
(a)
Interpretation:
The dynamic model of the given process is to be described along with the degree of freedom analysis.
Concept introduction:
For chemical processes, dynamic models consisting of ordinary differential equations are derived through unsteady-state conservation laws. These laws generally include mass and energy balances.
The process models generally include algebraic relationships which commence from
The degree of freedom analysis of a process model ensures that its model equations are solvable. The expression to calculate the degree of freedom is:
Here,
Answer to Problem 2.1E
The dynamic model for the given process is:
Degree of analysis results in 4 degrees of freedom for the given process.
Explanation of Solution
Given information:
A process model containing two input streams of the same liquid is stirred perfectly in a constant-volume tank. For each of the streams, the temperature and flowrate vary with time.
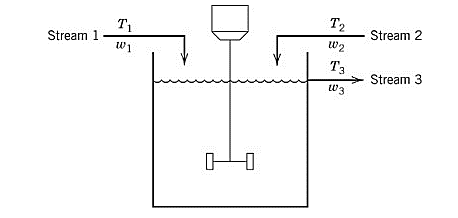
Assume that both the input streams come from the upstream units and their flowrates and temperature are known as the function of time.
For the given tank, apply the degree of freedom analysis. The parameters taken for the given process are the density
Variables taken for the given process model are
For the given process model, there will be 1 independent equation for the energy balance. Thus,
Use equation (1) to calculate the degree of freedom as:
Therefore, the degree of freedom analysis results in 4 degree of freedom for the given process model.
For the tank system given, write the overall mass balance equation as:
Here,
Substitute above equation in equation (2) as:
It is assumed that the tank is a constant-volume system and its density does not vary with time and temperature, thus,
Therefore, the mass balance on the given process model gives,
Now, apply energy balance and write its equation as the total energy of the tank is conserved.
Substitute equation (3) in above equation and simplify as:
Substitute equation (4) in the above equation and simplify the expression to get the dynamic model of the given process as:
(b)
Interpretation:
The derived dynamic model of the system is to be simplified by eliminating any algebraic equations.
Concept introduction:
For chemical processes, dynamic models consisting of ordinary differential equations are derived through unsteady-state conservation laws. These laws generally include mass and energy balances.
The process models generally include algebraic relationships which commence from thermodynamics, transport phenomena, chemical kinetics, and physical properties of the processes.
Answer to Problem 2.1E
The simplified dynamic model of the given system is:
Explanation of Solution
Given information:
A process model containing two input streams of the same liquid is stirred perfectly in a constant-volume tank. For each of the streams, the temperature and flowrate vary with time.
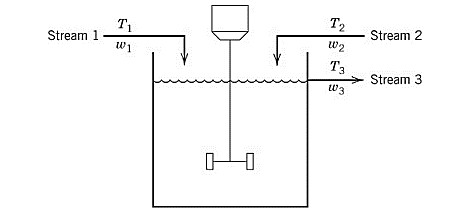
Assume that both the input streams come from the upstream units and their flowrates and temperature are known as the function of time.
From part (a), the dynamic model of the given process is derived as:
Now, let the reference temperature for the system is kept constant. So,
Want to see more full solutions like this?
Chapter 2 Solutions
EBK PROCESS DYNAMICS+CONTROL
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





