
Concept explainers
Answer the following questions about esmolol, a drug used to treat high blood pressure sold under the trade name Brevibloc.
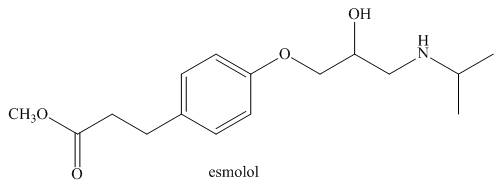
a. Label the most acidic hydrogen atom in esmolol.
b. What products are formed when esmolol is treated with
c. What products are formed when esmolol is treated with
d. Label all
e. Label the only triagonal pyramidal atom.
f. Label all C’s that bear
(a)
Interpretation: The most acidic hydrogen in the compound esmolol is to be labeled.
Concept introduction: The most acidic hydrogen
Answer to Problem 2.71P
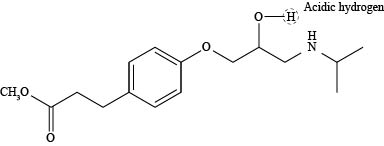
The labeling of the most acidic hydrogen present in the compound esmolol is shown below.
Explanation of Solution
The most acidic hydrogen in the compound esmolol is shown as,

Figure 1
According to the structure of esmolol, there are three elements present in the compound to which protons are bonded. The acidity increases while moving left to right in the period due to an increase in the electronegativity of the elements. Thus, oxygen atom is located at the extreme right position in the periodic table and is highly electronegative than carbon and nitrogen. Thus, the proton that is attached to oxygen atom is the most acidic one as shown above.
The labeling of the most acidic hydrogen present in the compound esmolol is shown in Figure 1.
(b)
Interpretation: The products formed by the reaction of esmolol with
Concept introduction: An atom or a group of atoms that shows characteristic physical and chemical properties are collectively known as functional groups. The functional group is the most reactive part present in the molecule. The main functional groups are
According to Bronsted-Lowry theory, the species that easily accept the proton is known as base and the species that easily donate the proton is known as acid. The reaction of an acid with a base always leads to the formation of conjugate acid and base.
Answer to Problem 2.71P
The products formed by the reaction of esmolol with
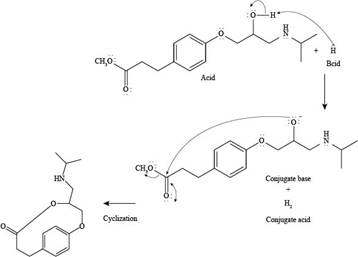
Explanation of Solution
The reaction of esmolol with
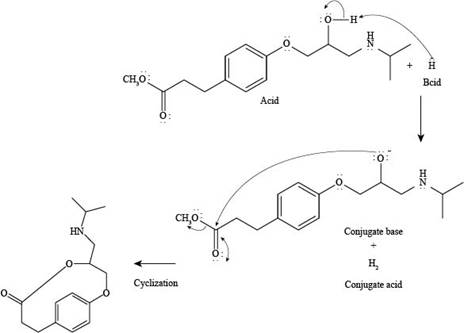
Figure 2
In the above reaction, first of all,
The products formed by the reaction of esmolol with
(c)
Interpretation: The products formed by the reaction of esmolol with
Concept introduction: An atom or a group of atoms that shows characteristic physical and chemical properties are collectively known as functional groups. The functional group is the most reactive part present in the molecule. The main functional groups are
According to Bronsted-Lowry theory, the species that easily tends to accept the proton is known as base and the species that easily donate the proton is known as acid. The reaction of an acid with a base always leads to the formation of conjugate acid and base.
Answer to Problem 2.71P
The products formed by the reaction of esmolol with
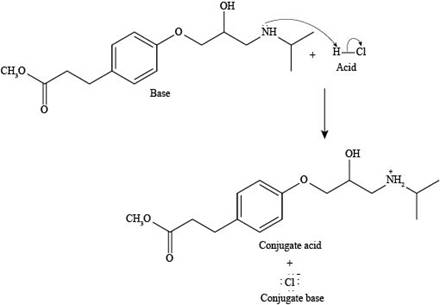
Explanation of Solution
The reaction of esmolol with
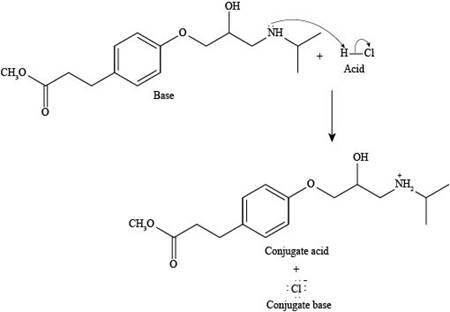
Figure 3
In the above reaction, a proton of
The products formed by the reaction of esmolol with
(d)
Interpretation: The
Concept introduction: The intermixing of
Answer to Problem 2.71P
The labeling of
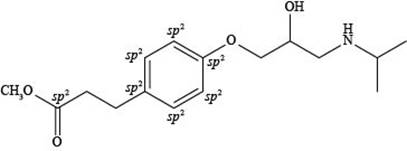
Explanation of Solution
The labeling of
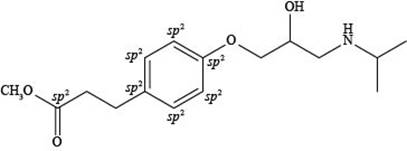
Figure 4
According to the structure of esmolol, there are total seven
The labeling of
(e)
Interpretation: The triagonal pyramidal atom present in the compound, esmolol is to be labeled.
Concept introduction: The atoms that possess
Answer to Problem 2.71P
The labeling of triagonal pyramidal atom present in the compound, esmolol is shown below.
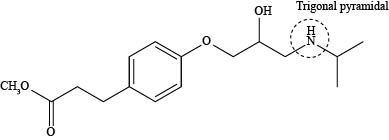
Explanation of Solution
The labeling of triagonal pyramidal atom present in the compound, esmolol is shown as,
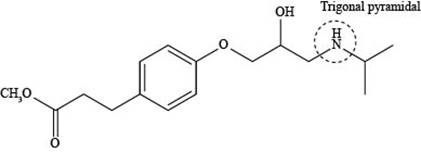
Figure 5
According to the structure of esmolol, nitrogen atom is present which is bonded to two carbon atoms and one hydrogen atom along with lone pair of electrons. This bonding of atoms with nitrogen results in the formation of
The labeling of triagonal pyramidal atom present in the compound, esmolol is shown in Figure 5.
(f)
Interpretation: All
Concept introduction: The
Answer to Problem 2.71P
The labeling of all
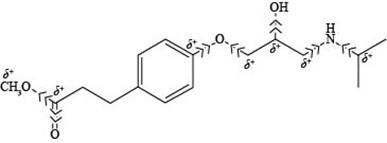
Explanation of Solution
The labeling of all

Figure 6
According to the structure of esmolol, the carbon atoms that are attached to nitrogen and oxygen possess
The labeling of all
Want to see more full solutions like this?
Chapter 2 Solutions
ORG.CHEMISTRY CONNECT ACCESS>CUSTOM<
- Rank the given compounds in order of decreasing basicity. 1=most basic, 4=least basicarrow_forwardcompared to HCO3-, H2co3 has a A Stronger conjugated base B Weaker conjugated base C Higher ka D Lower ka E Both b and carrow_forwardExplain why HC = CH is more acidic than CH3CH3, even though the C - H bond in HC = CH has a higher bond dissociation energy than the C - H bond in CH3CH3arrow_forward
- Nepheliosyne B is a novel acetylenic fatty acid isolated from a New Caledonian marine sponge. (a) Label the most acidic H atom. (b) Which carbon–carbon σ bond is shortest? (c) How many degrees of unsaturation does nepheliosyne B contain? (d) How many bonds are formed from Csp–Csp3? (e) Label each triple bond as internal or terminal.arrow_forwardCan someone please rank the hydrogen's on the third carbon from most acidic to least acidic?arrow_forward1.(a) Which of the following groups has the LOWEST IUPAC priority?(A) CH3 (B) NH2 (C) OH (D) COOH (E) Br (b)Which of the following corresponds to the strongest acid?(A) (CF3)3C-COOH (B) (CF3)3 C-OH(C) CH3COOH (D) CH3OH(E) HOCH2CH3arrow_forward
- Rank the following from strongest acid to weakest acid: CH3CH2N+H3 CH3CH=N+H2 CH3C=N+Harrow_forwardQuinapril (trade name Accupril) is a drug used to treat hypertension and congestive heart failure.a.Identify the functional groups in quinapril. b.Classify any alcohol, amide, or amine as 1°, 2°, or 3°. c. At which sites can quinapril hydrogen bond to water? d.At which sites can quinapril hydrogen bond to acetone [(CH3)2CO]? e. Label the most acidic hydrogen atom. f.Which site is most basic?arrow_forwardPropranolol is an antihypertensive agent – that is, it lowers blood pressure. (a) Which proton in propranolol is most acidic? (b) What products are formed when propranolol is treated with NaH? (c) Which atom is most basic? (d) What products are formed when propranolol is treated with HCl?arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
