
Concept explainers
Write a structural formula for the principal organic product or products of each of the
following reactions:
Propanoyl chloride and sodium propanoate
Butanoyl chloride and benzyl alcohol
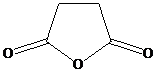 and water
and water
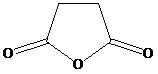 and aqueous sodium hydroxide to give
and aqueous sodium hydroxide to give
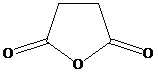 and aqueous ammonia to give
and aqueous ammonia to give
Methyl benzoate and excess phenylmagnesium bromide, then
Acetic anhydride and
Ethyl phenylacetate and lithium aluminum hydride, then
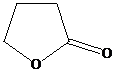 and aqueous sodium hydroxide to give
and aqueous sodium hydroxide to give
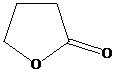 and aqueous ammonia
and aqueous ammonia
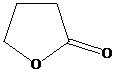 and lithium aluminum hydride, then
and lithium aluminum hydride, then
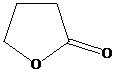 and excess methylmagnesium bromide, then
and excess methylmagnesium bromide, then
Ethyl phenylacetate and methylamine
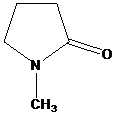 and aqueous sodium hydroxide to give
and aqueous sodium hydroxide to give
 and aqueous hydrochloric acid, heat to give
and aqueous hydrochloric acid, heat to give
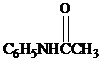 and aqueous hydrochloric acid, heat to give
and aqueous hydrochloric acid, heat to give
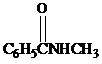 and aqueous sulfuric acid, heat to give
and aqueous sulfuric acid, heat to give
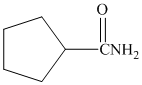 and
and
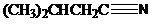 and aqueous hydrochloric acid, heat
and aqueous hydrochloric acid, heat
Propanenitrile and methylmagnesium bromide, then
Interpretation:
Structural formulae of the principal organic products of the given reactions are to be written.
Concept Introduction:
As carboxylic acid derivatives contains a carbonyl group, they undergo reactions typical of carbonyl compounds.
Reactivity of carboxylic acid derivative toward nucleophilic acyl substitution depends on its structure. Acid chlorides are most reactive, followed by anhydrides, esters and amides.
The reactivity order can be explained on the basis of resonance stabilization of the carbonyl group by the acyl substituent.
Nucleophilic substitution reactions of carboxylic acid derivatives generally proceed by an addition-elimination mechanism involving a tetrahedral intermediate.
Answer to Problem 31P
Solution:
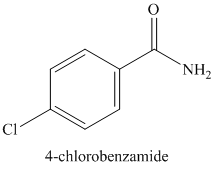
e)
f)
g)
h)
i)
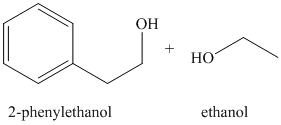
j)
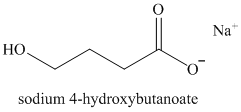
k)
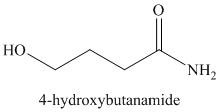
l)
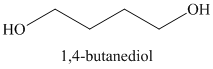
m)
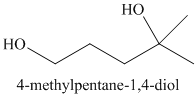
n)
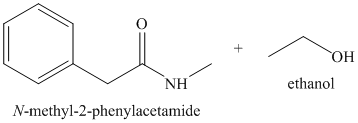
o)
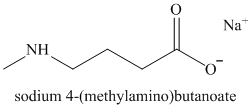
p)
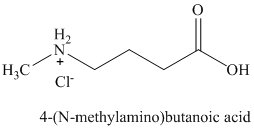
q)
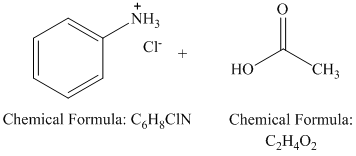
r)
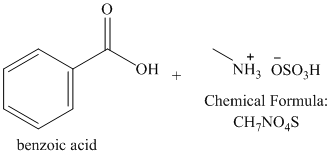
s)

t)
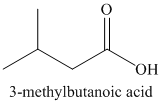
u)
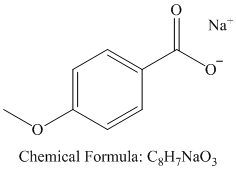
v)
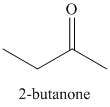
Explanation of Solution
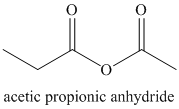
a) Propanoyl chloride and sodium propanoate:
The propanoate anion acts as a nucleophile, adding to the carbonyl carbon from propanoyl chloride. Chloride is displaced in the next step, reforming the carbonyl to yield the mixed anhydride acetic propanoic anhydride.
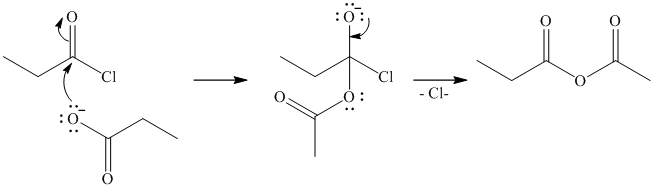
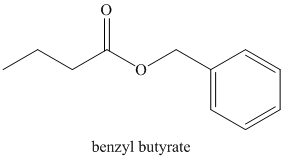
b) Butanoyl chloride and benzyl alcohol:
The oxygen of benzyl alcohol adds to the electrophilic carbon of the carbonyl group in butanoyl chloride. Loss of the chloride and deprotonation yields benzyl butyrate.
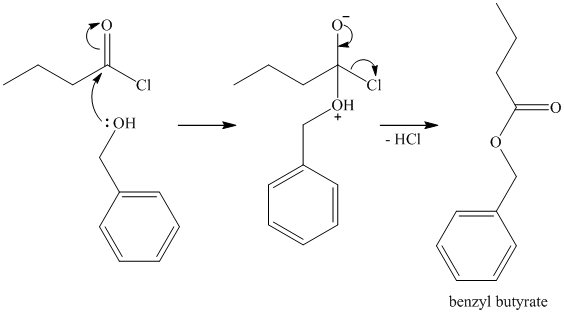
c)
Ammonia is the nucleophile in this reaction and adds to the carbonyl carbon of
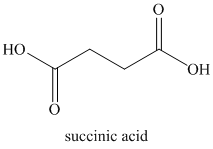
d) Succinic anhydride and water:
Succinic anhydride hydrolyzes to succinic acid
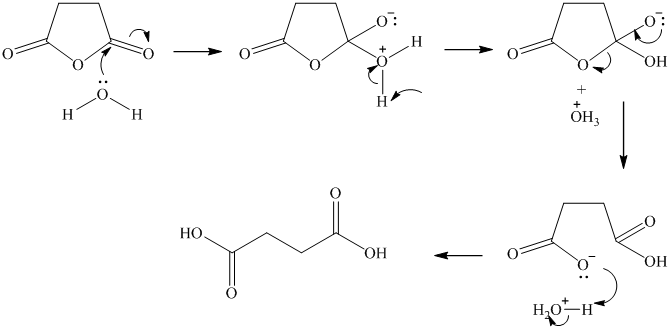
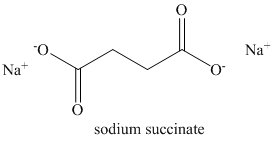
e) Succinic anhydride and aqueous sodium hydroxide to give
Succinic anhydride undergoes hydrolysis in basic aqueous conditions. The sodium salt of the acid is formed under these conditions.
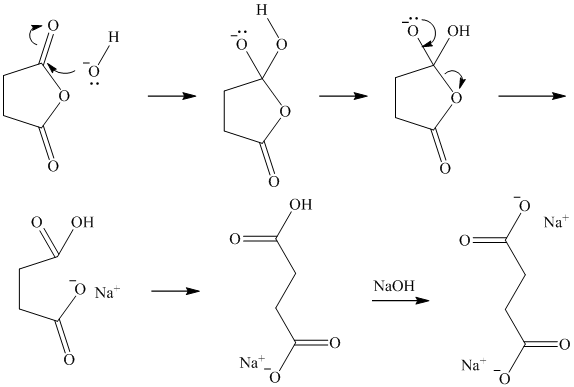
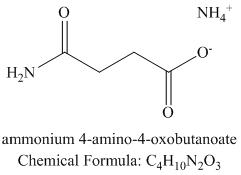
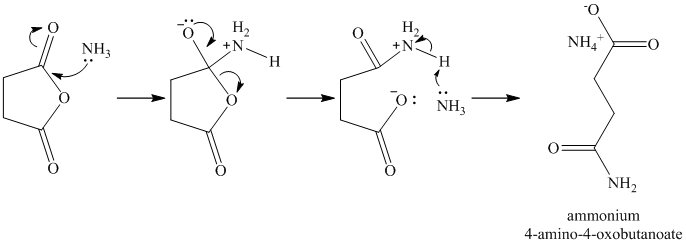
f) Succinic anhydride and aqueous ammonia to give
Ammonia attacks one of the carbonyl carbons. The bond between this carbonyl and the acyl group breaks. An amide group if formed with another molecule of ammonia extracting a proton. Cleavage of the bond between this carbonyl and the anhydride oxygen results in a carboxylate group at the other end of the carbon chain. This forms an ammonium salt.
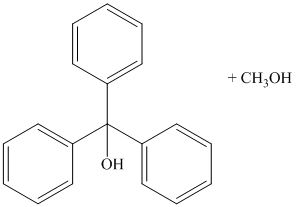
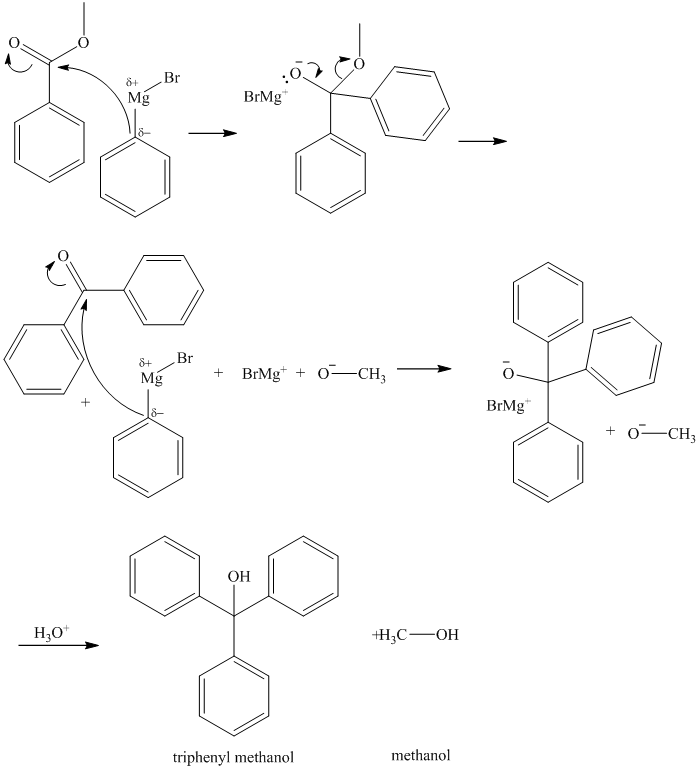
g) Methyl benzoate and excess phenylmagnesium bromide, then
Grignard reagents are good nucleophiles and attack the electrophilic carbonyl carbon in esters. A carbon-carbon bond is formed between the carbonyl carbon and the phenyl group from the Grignard reagent, phenylmagnesium bromide. Loss of methoxy group from the ester leads to initial formation of diphenyl ketone. As an excess of phenylmagnesium bromide is used, another phenyl group is added to the initially formed ketone to produce triphenylmethoxy ion. Acidic work up yields triphenyl methanol.
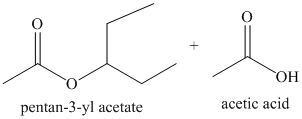
h) Acetic anhydride and
The alcohol acts as a nucleophile and adds to one of the carbonyl carbons from the anhydride. The loss of acyl group followed by deprotonation of alcohol oxygen yields the ester
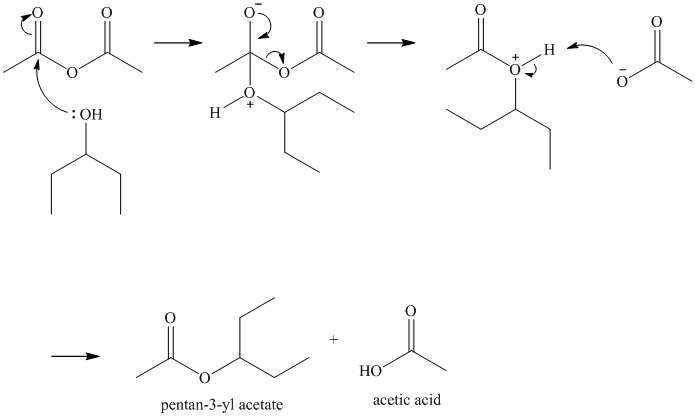
Ethyl phenylacetate and lithium aluminum hydride, then
Lithium aluminum hydride is a source of hydride ion. It is used for reduction of a variety of functional groups including esters. The hydride functions as a strong nucleophile, and initially adds to the carbonyl carbon of the ester. The loss of ethoxy group gives
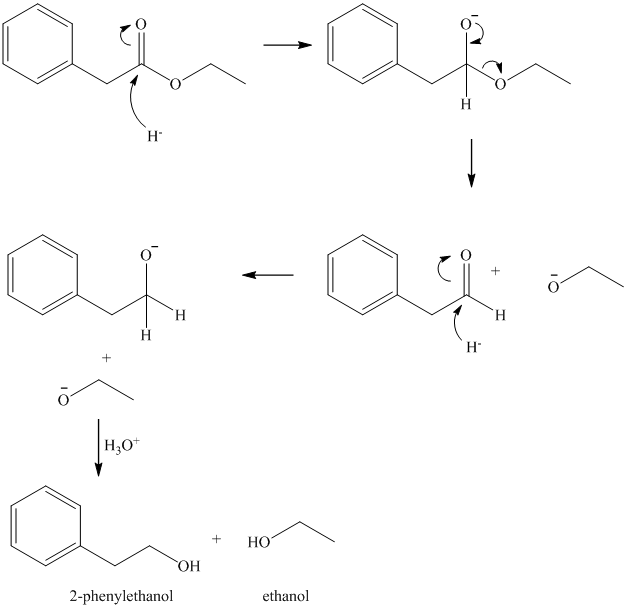
As the acetaldehyde also contains a carbonyl group, addition of a second hydride to it converts it to
j)
Lactones are cyclic esters. They undergo hydrolysis when treated with aqueous solution of bases like
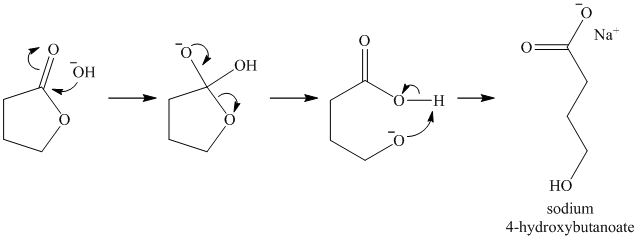
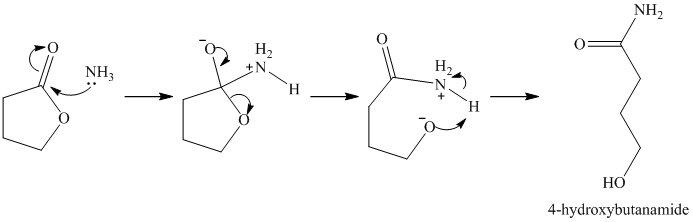
k)
Ammonia molecule adds to the carbonyl carbon, and forms an amide group on deprotonation. The ester bond breaks, forming an alkoxy group at the other end of the carbon chain. Protonation of this forms an alcoholic
l)
Lithium aluminum hydride reduces lactones to diols. A hydride ion adds to the carbonyl carbon of the lactone. Breaking of the ester
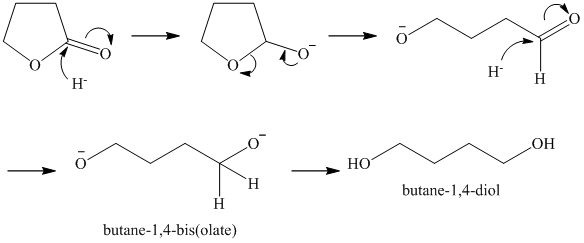
Acidic work up converts this into
m)
Like open chain esters, lactose also reacts with two moles of Grignard reagent per mole lactone. Because a lactone is in internal, cyclic ester, a diol is formed instead of two molecules of alcohol. The reaction starts with the methyl group from the Grignard reagent adding to the carbonyl carbon. This results in breaking of the lactone ring, with a ketone group at one end and an alkoxy group at the other end of the carbon chain. The ketone group reacts with another molecule of methylmagnesium bromide and is converted to a secondary alcohol on acidic work up. Therefore, this reaction gives the product
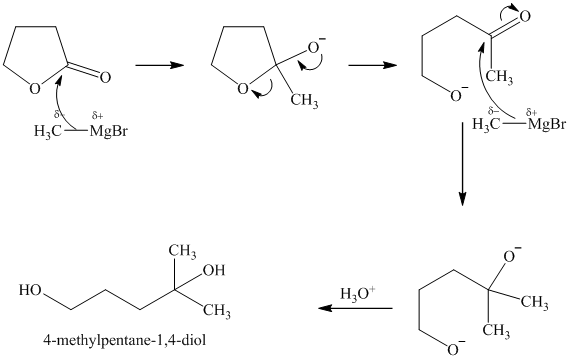
n) Ethyl phenylacetate and methylamine (
This reaction alo is addition-elimination, resulting in the formation of an amide and an alcohol. Methyl amine acts as a nucleophile and adds to the carbonyl carbon of the ester. Loss of the ethoxy group, and deprotonation of the protonated amide yields
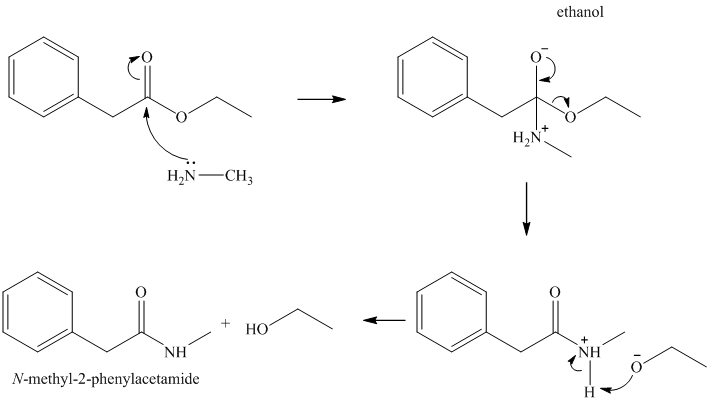
o)
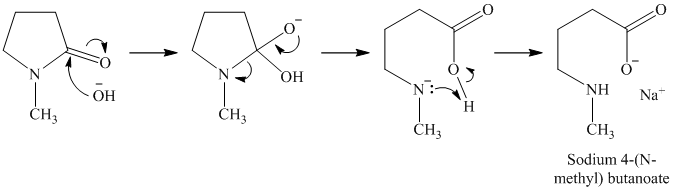
p)
Lactams are poor electrophiles because of the planar,
Therefore, the product of the reaction is the hydrochloride of
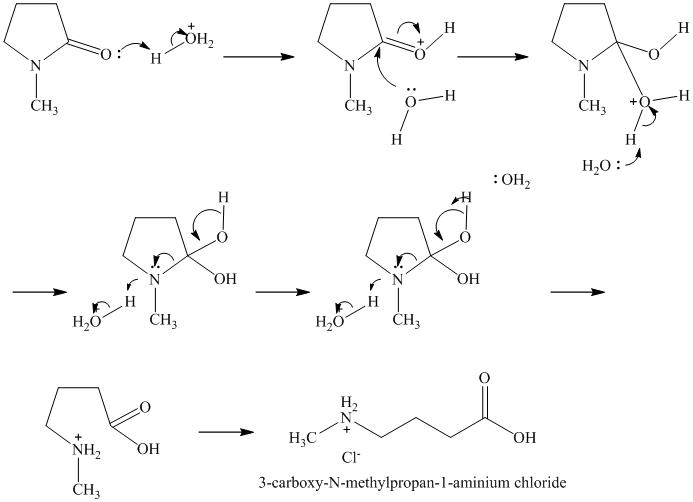
q)
Acid hydrolysis of an amide results in the formation of a carboxylic acid and an amide (as amide hydrochloride). The amide group is activated by protonation of the oxygen. Addition of water molecule to the carbonyl carbontakes place. Subsequent proton transfers and breaking of the amide bond yields acetic acid and aniline hydrochloride.
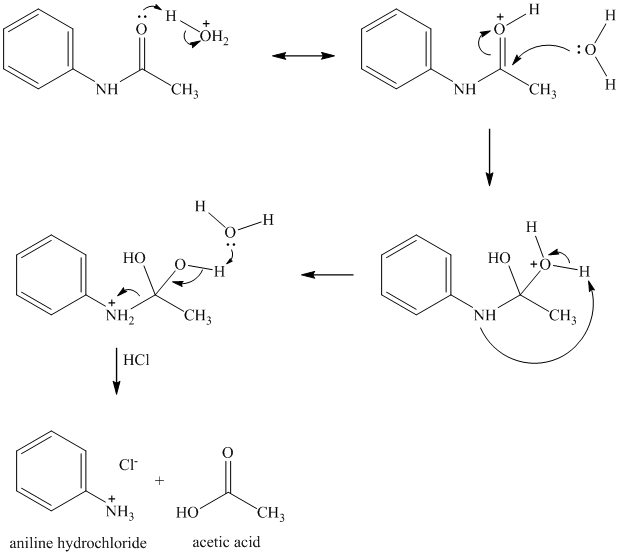
r)
Acidic hydrolysis of
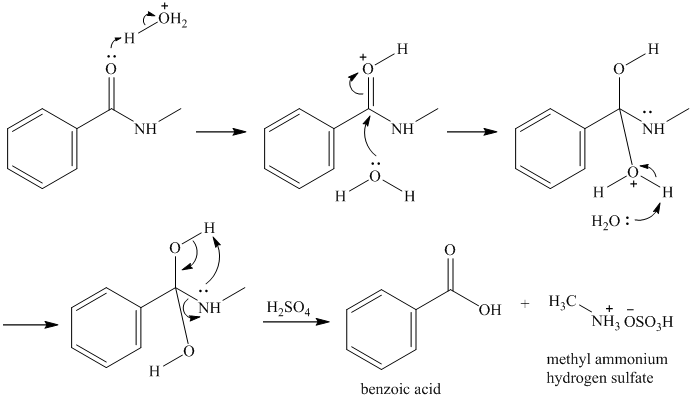
s) Cyclopentanecarboxamideand
The reaction starts with the nucleophilic oxygen of amide attacking the phosphorus atom. Another oxygen bonded to this phosphorus deprotonates the amine group, with the bond pair moving to form the nitrile triple bond. The remaining proton attached to the nitrogen is extracted by another of the negatively charged oxygens in phosphorus pentoxide to give the product.
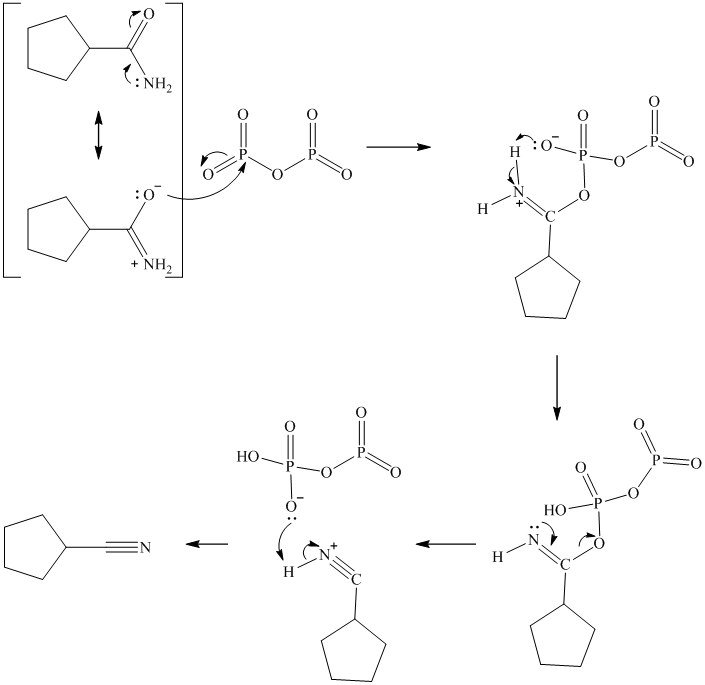
t)
Acid hydrolysis of nitriles is reverse reaction of the synthesis of nitrile from an amide. Under acidic conditions, the nitrile is initially hydrolyzed to an amide. The amide undergoes further hydrolysis to a carboxylic acid and ammonium salt.
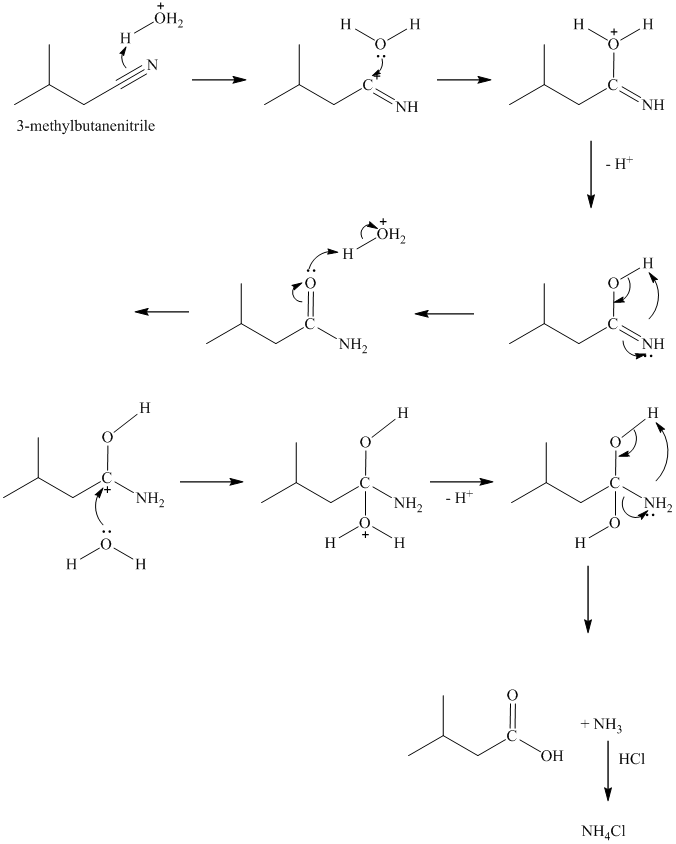
u)
Hydrolysis of nitrile initially forms an amide, with the hydroxide attacking the nitrile carbon. The amide formed undergoes further hydrolysis to form the carboxylate salt and ammonia.
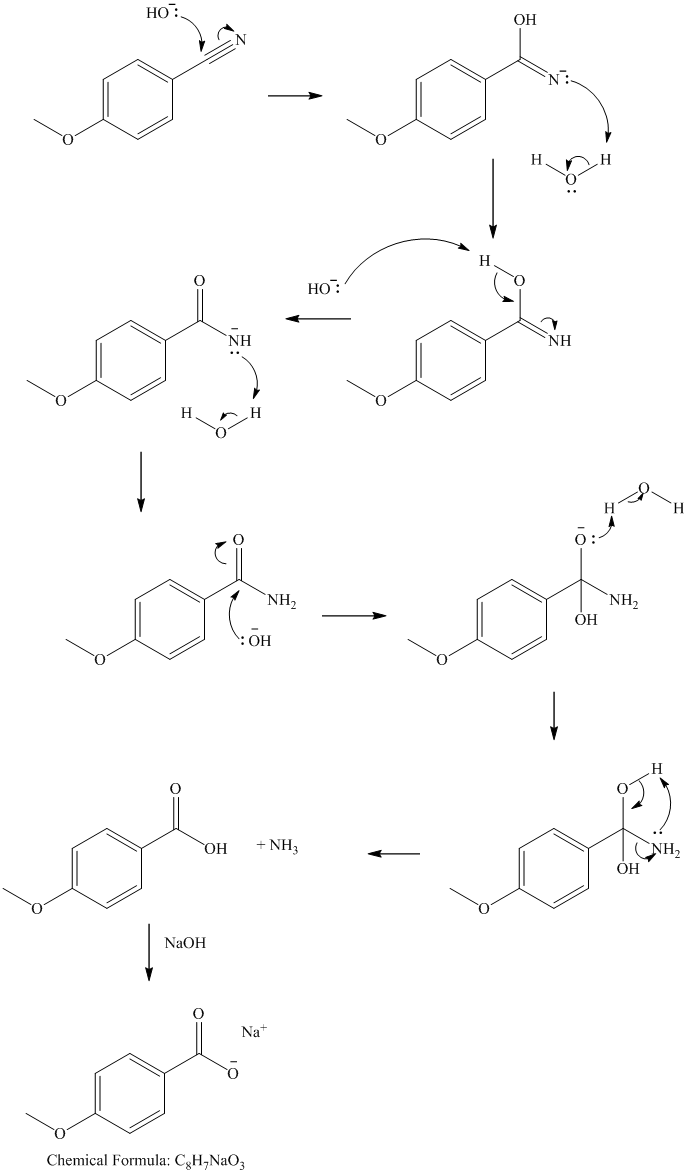
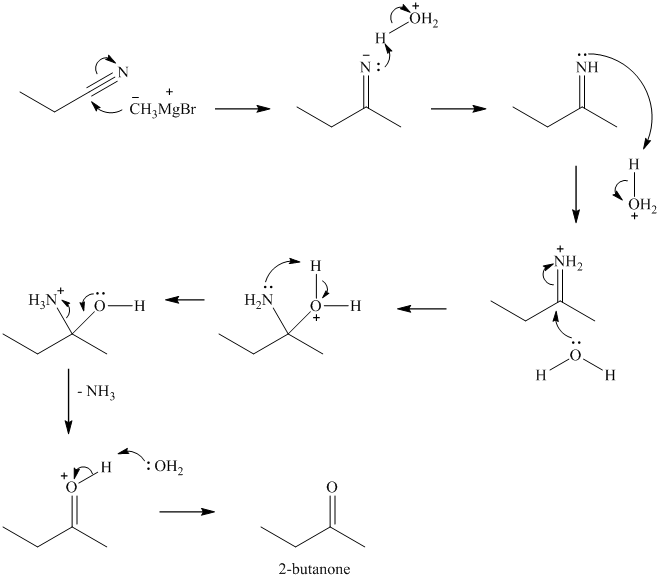
v) Propanenitrile and methylmagnesium bromide, then
The strongly basic methylmagnesium bromide attacks the nitrile carbon, forming an imine on protonation of the nitrogen. Imine then undergoes hydrolysis to ketone and ammonia.
Want to see more full solutions like this?
Chapter 20 Solutions
ORGANIC CHEMISTRY-W/STUD.SOLN.MAN.
Additional Science Textbook Solutions
General, Organic, and Biological Chemistry (3rd Edition)
The Organic Chem Lab Survival Manual: A Student's Guide to Techniques
Chemistry: Matter and Change
General Chemistry: Principles and Modern Applications (11th Edition)
- Claisen condensation between diethyl phthalate and ethyl acetate followed by saponification, acidification, and decarboxylation forms a diketone, C9H6O2. Propose structural formulas for compounds A and B and the diketone.arrow_forwardOutline syntheses of the following compounds, starting with triphenylphosphine, an alkyl halide and an aldehyde or ketone.a) 2-pentene b) Trans 1,2-diphenylethene c) Cyclohexylethenearrow_forwardWrite equations showing how to prepare each of the following from benzene or toluene and any necessary organic or inorganic reagents. If an ortho, para mixture is formed in any step of your synthesis, assume that you can separate the two isomers. (a) Isopropylbenzene (b) p-Isopropylbenzenesulfonic acid (c) 2-Bromo-2-phenylpropane (d) 4-tert-Butyl-2-nitrotoluene (e) m-Chloroacetophenone (f) p-Chloroacetophenone (g) 3-Bromo-4-methylacetophenone (h) 2-Bromo-4-ethyltoluene (i) 3-Bromo-5-nitrobenzoic acid (j) 2-Bromo-4-nitrobenzoic acid (k) 1-Phenyloctane (l) 1-Phenyl-1-octene (m) 1-Phenyl-1-octyne (n) 1,4-Di-tert-butyl-1,4-cyclohexadienearrow_forward
- Write the structure of the principal organic product formed in the reaction of 1-propanol with each of the following reagents:(a) Sulfuric acid (catalytic amount), heat at 140°C(b) Sulfuric acid (catalytic amount), heat at 200°C(c) Dimethyl sulfoxide (DMSO), oxalyl chloride [(COCl)2], triethylamine [N(CH2CH3)3](d) Pyridinium chlorochromate (PCC) in dichloromethane(e) Potassium dichromate (K2Cr2O7) in aqueous sulfuric acid, heat(f) Sodium amide (NaNH2)arrow_forward(a) Give chemical tests to distinguish between the following pairs of compounds :(i) Benzoic acid and Phenol (ii) Benzaldehyde and Acetophenone(b) An organic compound with molecular formula C5H10O does not reduce Tollen’s reagent but forms an addition compound with sodium hydrogen sulphite and gives a positive iodoform test. On vigorous oxidation, it gives ethanoic acid and propanoic acid. Identify the compound and write all chemical equations for the reactions.arrow_forwardProvide the reagents necessary to complete the following reactions.arrow_forward
- Write the equilibrium-constant expressions and obtainnumerical values for each constant in. (a) the basic dissociation of aniline, C6H5NH2. (b) the acidic dissociation of hypochlorous acid,HClO. (c) the acidic dissociation of methyl ammoniumhydrochloride, CH3NH3Cl. (d) the basic dissociation of NaNO2. (e) the dissociation of H3AsO3to H3O+and AsO33-. (f) the reaction of C2O42-with H2O to give H2C2O4and OH-. show solutionarrow_forwardWhat reactions and reagents can be used to make phenol from benzene if electrophilic aromatic substitution reactions are excluded and benzene is the only source of carbon?arrow_forwardWhich of the following sets of reagents would not be an acceptable method for the preparation of ethylacetate? acetic acid, ethanol, and an acid catalyst Acetic anhydride and ethanol Sodium acetate and ethanol Sodium acetate and ethyliodidearrow_forward
- Write the structure of the major organic product isolated from the reaction of 3-hexyne with (a) Hydrogen (2 mol), platinum (b) Hydrogen (1 mol), Lindlar palladium (c) Hydrogen chloride (1 mol) (d) Hydrogen chloride (2 mol) (e) Chlorine (1 mol) (f) Chlorine (2 mol) (g) Aqueous sulfuric acid, mercury(II) sulfate (h) Ozone followed by hydrolysisarrow_forwardExplain this statement: Although 2-methoxyacetic acid (CH3OCH2COOH) is a stronger acid than acetic acid (CH3COOH), p-methoxybenzoic acid (CH3OC6H4COOH) is a weaker acid than benzoic acid (C6H5COOH).arrow_forwardExplain this statement: Although 2-methoxyacetic acid (CH3OCH2COOH)is a stronger acid than acetic acid (CH3COOH), p-methoxybenzoic acid(CH3OC6H4COOH) is a weaker acid than benzoic acid (C6H5COOH).arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

