
(a)
Interpretation: The
Concept introduction:
Ocean acidification: The increase in concentration of carbon-dioxide in the atmosphere leads to ocean acidification. The amount is increasing day by day and this increase will lead to higher concentrations of dissolved
The concentration of hydronium ion is a key factor for many biochemical reactions. So variation may affect the organisms in the oceans.
The relationship between ocean
(a)
Answer to Problem 40GQ
The
Explanation of Solution
If the concentration of dissolved
The graph showing the fraction of species in the solution as a function of
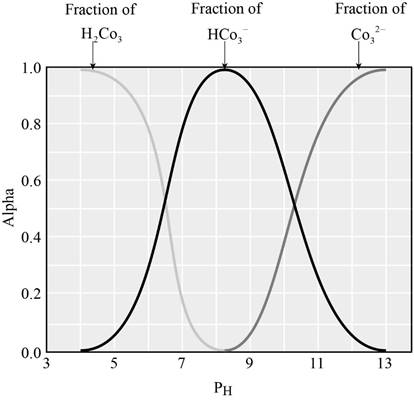
From the graph we can identify the
The fraction of dissociation will be 0.50 when the concentration of both the species will be equal.
The
(b)
Interpretation: The
Concept introduction:
Ocean acidification: The increase in concentration of carbon-dioxide in the atmosphere leads to ocean acidification. The amount is increasing day by day and this increase will lead to higher concentrations of dissolved
The concentration of hydronium ion is a key factor for many biochemical reactions. So variation may affect the organisms in the oceans.
The relationship between ocean
(b)
Answer to Problem 40GQ
The
Explanation of Solution
If the concentration of dissolved
The graph showing the fraction of species in the solution as a function of
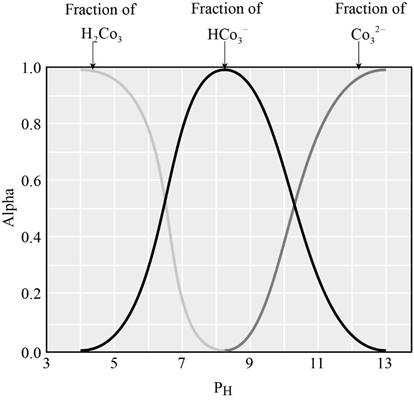
From the graph we can identify the
The fraction of dissociation will be 0.50 when the concentration of both the species will be equal.
The
(c)
Interpretation: The predominant species in the solution when the
Concept introduction:
Ocean acidification: The increase in concentration of carbon-dioxide in the atmosphere leads to ocean acidification. The amount is increasing day by day and this increase will lead to higher concentrations of dissolved
The concentration of hydronium ion is a key factor for many biochemical reactions. So variation may affect the organisms in the oceans.
The relationship between ocean
(c)
Answer to Problem 40GQ
The predominant species in the solution when the
Explanation of Solution
If the concentration of dissolved
The graph showing the fraction of species in the solution as a function of
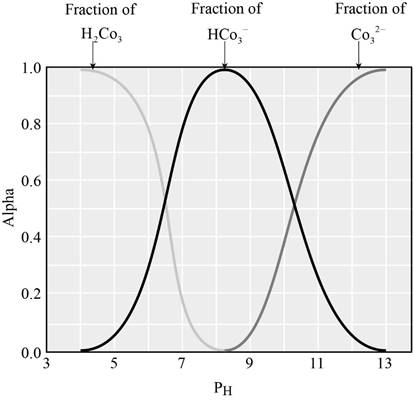
When the
From the graph it is clear that, the predominant species when the
(d)
Interpretation: The predominant species in the solution when the
Concept introduction:
Ocean acidification: The increase in concentration of carbon-dioxide in the atmosphere leads to ocean acidification. The amount is increasing day by day and this increase will lead to higher concentrations of dissolved
The concentration of hydronium ion is a key factor for many biochemical reactions. So variation may affect the organisms in the oceans.
The relationship between ocean
(d)
Answer to Problem 40GQ
The predominant species in the solution when the
Explanation of Solution
If the concentration of dissolved
The graph showing the fraction of species in the solution as a function of
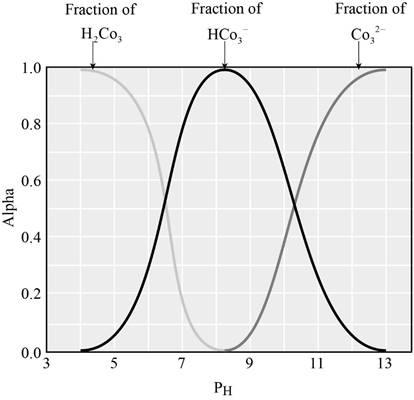
When the
From the graph it is clear that, the predominant species when the
Want to see more full solutions like this?
Chapter 20 Solutions
Bundle: Chemistry & Chemical Reactivity, Loose-Leaf Version, 9th + OWLv2, 4 terms (24 Months) Printed Access Card
- Sodium perchlorate, NaClO4, is produced by electrolysis of sodium chlorate, NaClO3. If a current of 2.50 103 A passes through an electrolytic cell, how many kilograms of sodium perchlorate are produced per hour?arrow_forwardWhich of the following reactions occur spontaneously as written, with the production of a measurable electric current? a I2+NaBrBr2+2NaI b Li+NaClLiCl+Na c Li++Na+NaLi d Ag+LiAg+Li+ e 2Li++F22LiFarrow_forwardWhat chemical is described as 1MH2SO4?arrow_forward
- Will the following reaction accur? Explain. Pka h2o: 14 pka C2h2:25 pka hcooh: 3.8arrow_forward(i) State the Pauling First Rule for predicting the pKa of oxoacid.Illustrate your answer with an equation. (ii) State the Pauling Second Rule for predicting the pKa of oxoacid.Illustrate your answer with an equationarrow_forwardPhosphate buffered saline (PBS) is a very common reagent in cell biology labs that do cell culture since is it osmotically stable for most eukaryotic cells. It is often prepared as 10X PBS (10.5 mM KH2PO4, 1.552 M NaCl, 29.7 mM Na2HPO4) to save on shelf space. How would you prepare 1 L of 10X PBS given 1M KH2PO4, NaCl (FW 58.44), 906mM Na2HPO4? a. What do you know about the forms of thechemicals you are given to prepare the 10X PBS and how do you know this? What should the final units for each of the 3 components be in your answer? What is the solvent for 10X PBS and how do you know this?arrow_forward
- Which hazard is not associated with the sodium fusion operation? Open flame Hot glassware Strongly acidic solution (6 M H2SO4) Highly reactive sodium metal Potentially sharp broken glasswarearrow_forwardAssign reasons for the following :(i) Transition metals and many of their compounds act as good catalysts.(ii) Transition metals generally form coloured compounds.arrow_forwardWhat is 4d?arrow_forward
- Define racemic mixture? And briefly explain the Racemic modification and its types?arrow_forwardA student found in a reference that the pka of a certain weak acid is 2.74. What is the ionization constant of the acid, ka?arrow_forwardxplain simply, by what process this transformation occur; Iodine-131 to Xenon-131arrow_forward
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning


