
Figure 20-6 is the
(a)
The temperature of the gas at B, if the temperature at A is
Answer to Problem 52SP
Solution:
Explanation of Solution
Given data:
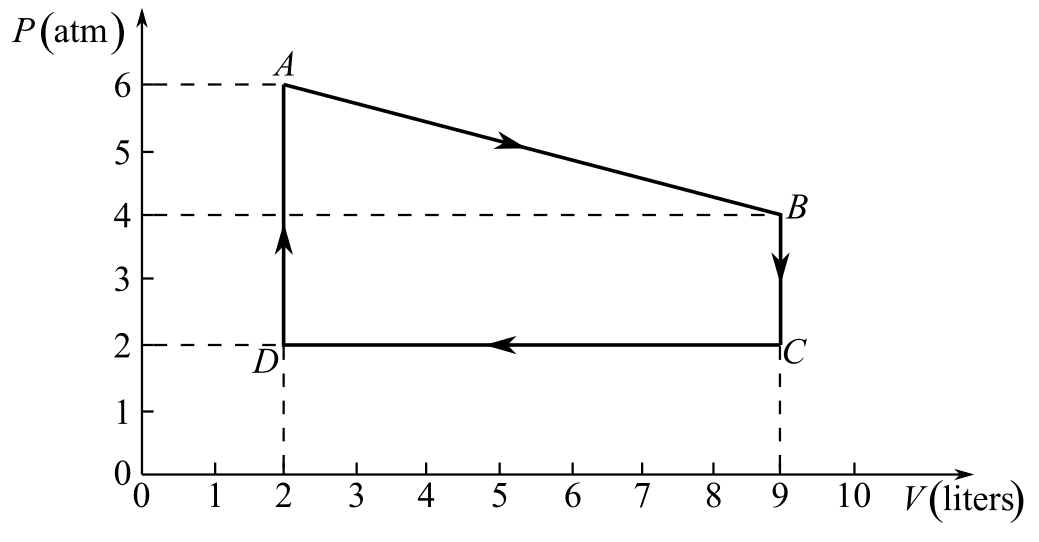
Refer to Fig. 20-6.
The temperature at A is
The mass of the ideal gas enclosed is
The specific heat of the gas at constant volume is
The gas follows the process A to B in the thermodynamic cycle shown in Fig. 20-6.
Formula used:
The gas equation between initial and final condition of a gas can be written as,
Here,
The formula for the conversion of the initial temperature of a gas from the Celsius scale to the Kelvin scale is,
Here,
The area of a trapezium is calculated by the formula,
Here,
The work done in a thermodynamic process is given by the area under the line representing the process in the pressure-volume diagram.
Here,
Explanation:
Draw the thermodynamic cycle diagram given in Fig- 20.6.
Recall the expression for the conversion of temperature at A from Celsius to Kelvin.
Here,
Substitute
Refer to the diagram and write the values of pressure and volume at points A and B, respectively.
And,
Here,
Recall the gas equation between points A and B.
Here,
Substitute
Conclusion:
The temperature at point B is
(b)
The value of
Answer to Problem 52SP
Solution:
Explanation of Solution
Given Data:
Refer to Fig. 20-6.
The temperature at A is
The mass of the ideal gas enclosed is
The specific heat of the gas at constant volume is
Formula Used:
The formula for change in internal energy is,
Here,
The formula for conversion of temperature of gas from Kelvin scale to Celsius scale is,
Here,
Explanation:
Draw the thermodynamic cycle diagram given in Fig- 20.6.

Calculate the temperature at point B in Celsius.
Here,
Substitute
Calculate the change in temperature from A to B.
Substitute
Recall the formula for change in internal energy.
Substitute
Conclusion:
The value of
(c)
The value of
Answer to Problem 52SP
Solution:
Explanation of Solution
Given Data:
Refer to Fig. 20-6.
The temperature at A is
The mass of the ideal gas enclosed is
The specific heat of the gas at constant volume is
Formula Used:
The area of a trapezium is calculated by the formula,
Here,
The work done in a thermodynamic process is given by the area under the line representing the process in the pressure-volume diagram.
Here,
Explanation:
Draw the thermodynamic cycle diagram given in Fig- 20.6.

Understand that the work done in the thermodynamic process AB is equal to the area of the pressure-volume diagram under the line AB.
Draw the thermodynamic cycle diagram showing the area under the line AB.
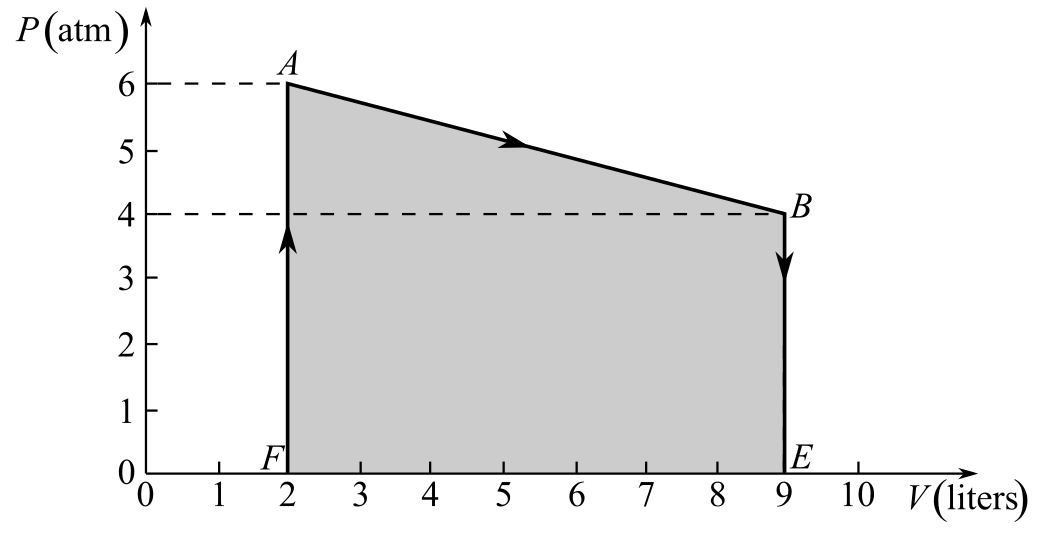
Here, the points E and F are as shown in the figure and the work done during the process AB is represented by the area under the line AB, which is equal to the area of the trapezium ABEF.
Refer to the figure and write the values of the lengths of sides AF, BE, and EF.
Recall the expression for the area of trapezium ABEF to calculate the area under the line AB in order to calculate the work done in the process AB.
Here,
Substitute
Recall the expression for the net-work done in a thermodynamic process in terms of the area of the pressure-volume diagram.
Substitute
The work done from A to B is
Conclusion:
The value of
(d)
The value of
Answer to Problem 52SP
Solution:
Explanation of Solution
Given Data:
Refer to Fig. 20-6.
The temperature at A is
The mass of the ideal gas enclosed is
The specific heat of the gas at constant volume is
Formula Used:
The first law of thermodynamics for a process is written as,
Here,
Explanation:
Draw the thermodynamic cycle diagram given in Fig- 20.6.

Recall the expression for the first law of thermodynamics for the process AB.
Substitute
Conclusion:
The value of
Want to see more full solutions like this?
Chapter 20 Solutions
Schaum's Outline of College Physics, Twelfth Edition (Schaum's Outlines)
- An ideal gas initially at 300 K undergoes an isobaric expansion at 2.50 kPa. If the volume increases from 1.00 m3 to 3.00 m3 and 12.5 kJ is transferred to the gas by heat, what are (a) the change in its internal energy and (b) its final temperature?arrow_forwardA sample of a monatomic ideal gas occupies 5.00 L at atmospheric pressure and 300 K (point A in Fig. P17.68). It is warmed at constant volume to 3.00 atm (point B). Then it is allowed to expand isothermally to 1.00 atm (point C) and at last compressed isobarically to its original state. (a) Find the number of moles in the sample. Find (b) the temperature at point B, (c) the temperature at point C, and (d) the volume at point C. (e) Now consider the processes A B, B C, and C A. Describe how to carry out each process experimentally. (f) Find Q, W, and Eint for each of the processes. (g) For the whole cycle A B C A, find Q, W, and Eint. Figure P17.68arrow_forwardFor a temperature increase of 10 at constant volume, what is the heat absorbed by (a) 3.0 mol of a dilute monatomic gas; (b) 0.50 mol of a dilute diatomic gas; and (c) 15 mol of a dilute polyatomic gas?arrow_forward
- An amount of n moles of a monatomic ideal gas in a conducting container with a movable piston is placed in a large thermal heat bath at temperature T1 and the gas is allowed to come to equilibrium. After the equilibrium is leached, the pressure on the piston is lowered so that the gas expands at constant temperature. The process is continued quasi-statically until the final pressure is 4/3 of the initial pressure p1 . (a) Find the change in the internal energy of the gas. (b) Find the work done by the gas. (c) Find the heat exchanged by the gas, and indicate, whether the gas takes in or gives up heat.arrow_forwardAn ideal gas has a pressure of 0.50 atm and a volume of 10 L. It is compressed adiabatically and quasi-statically until its pressure is 3.0 atm and its volume is 2.8 L. Is the monatomic, diatomic, or polyatomic?arrow_forwardA monatomic ideal gas undergoes a quasi-static adiabatic expansion in which its volume is doubled. How is the pressure of the gas changed?arrow_forward
- Two moles of a monatomic ideal gas such as oxygen is compressed adiabatically and reversibly from a state (3 atm, 5 L) to a state with a pressure of 4 atm. (a) Find the volume and temperature of the final state. (b) Find the temperature of the initial state. (c) Find work done by the gas in the process. (d) Find the change in internal energy in the process. Assume Cv=5R and Cp=Cv+R for the diatomic ideal gas in the conditions given.arrow_forwardSuppose a monatomic ideal gas is changed from state A to state D by one of the processes shown on the PV diagram.where P1 = 3.10 and P2 = 6.20. Find the total work done on the gas if it follows the constant-volume path AB followed by the constant-pressure path BCD.arrow_forwardA diatomic ideal gas at pressure p and volume V is expanding to three times its initial volume under constant pressure. W=2pV. a) If the initial temperature equals T, express the final temperature Tf in terms of the original temperature T. b) In terms of p and V, calculate the expression of the heat Q flowing into the gas. c) In terms of p and V, calculate the expression of the change in internal energy ΔU.arrow_forward
 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning


