
Concept explainers
(a)
Interpretation: The combination of diene and dienophile that undergoes Diels-Alder reaction to give the given adduct, has to be found.
Concept Introduction:
Diels-Alder reaction:
It is the reaction of conjugated dienes with double or triple bonded compounds which are known as “dienophiles”. The reaction is a
Example:
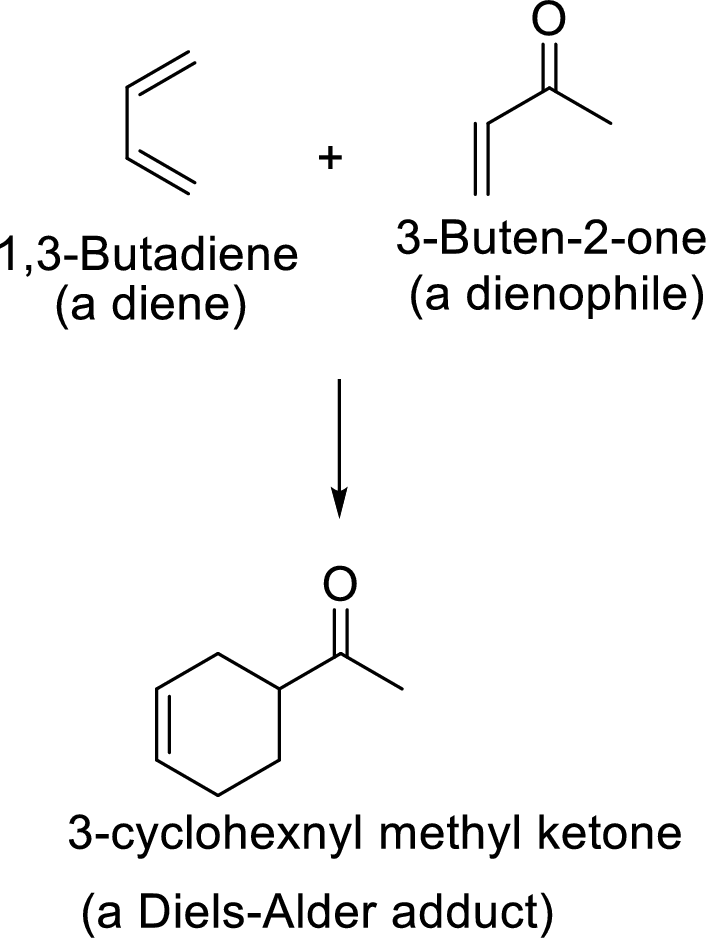
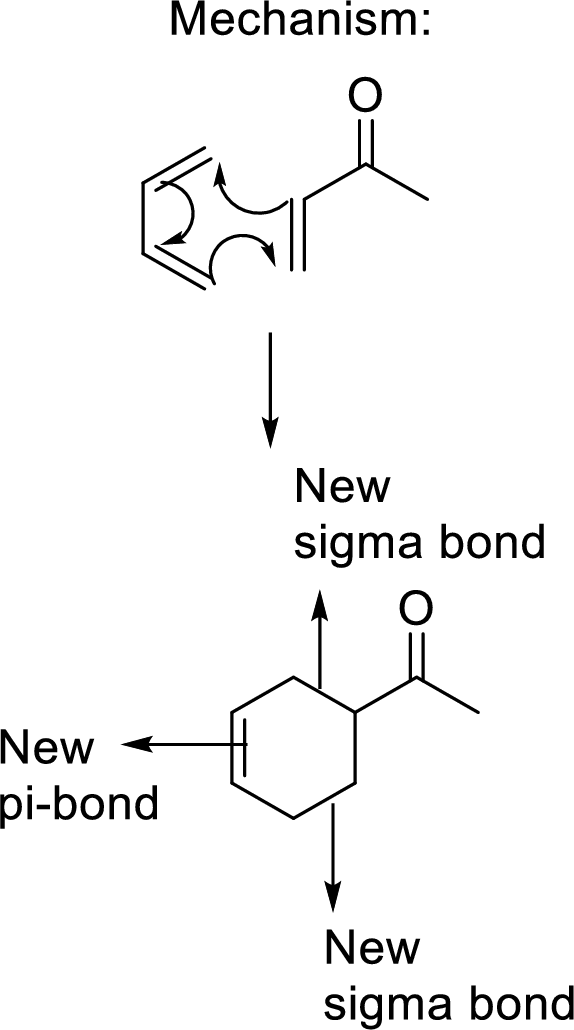
This mechanism shown that three
(b)
Interpretation: The combination of diene and dienophile that undergoes Diels-Alder reaction to give the given adduct, has to be found.
Concept Introduction:
Diels-Alder reaction:
It is the reaction of conjugated dienes with double or triple bonded compounds which are known as “dienophiles”. The reaction is a
Example:
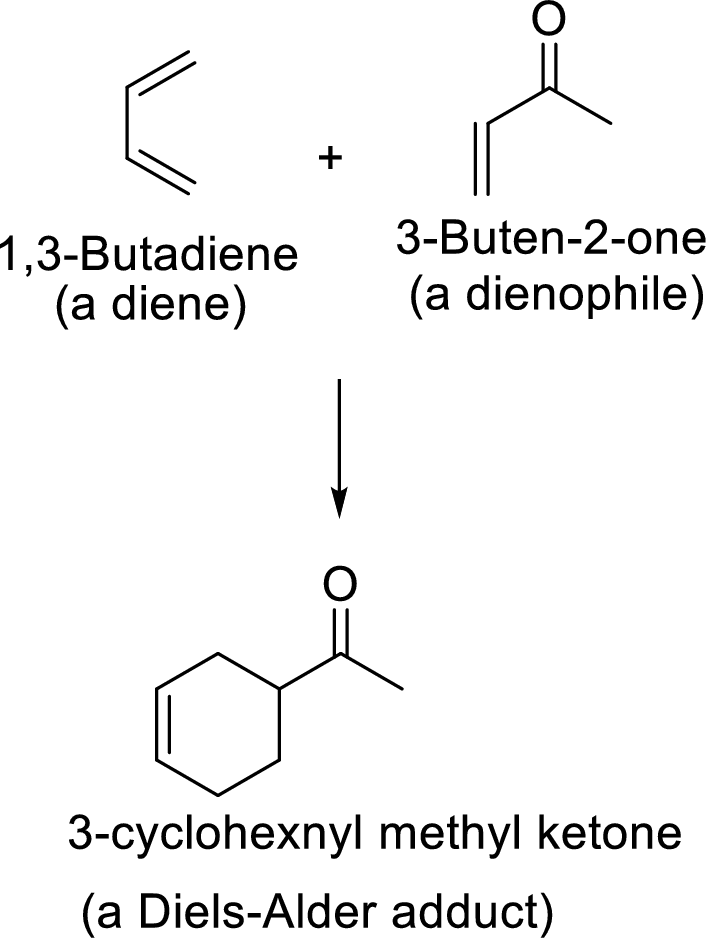
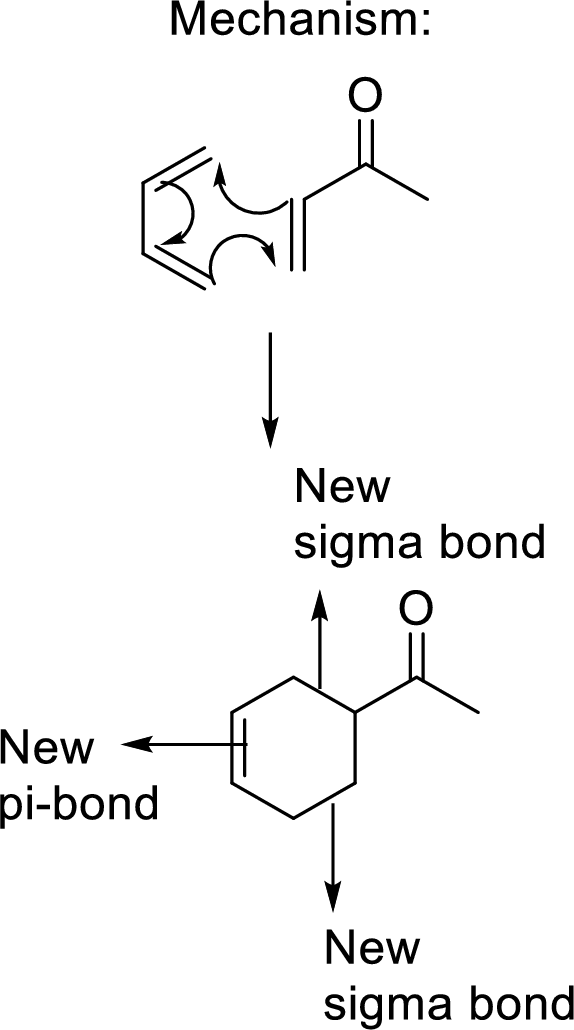
This mechanism shown that three
(c)
Interpretation: The combination of diene and dienophile that undergoes Diels-Alder reaction to give the given adduct, has to be found.
Concept Introduction:
Diels-Alder reaction:
It is the reaction of conjugated dienes with double or triple bonded compounds which are known as “dienophiles”. The reaction is a
Example:
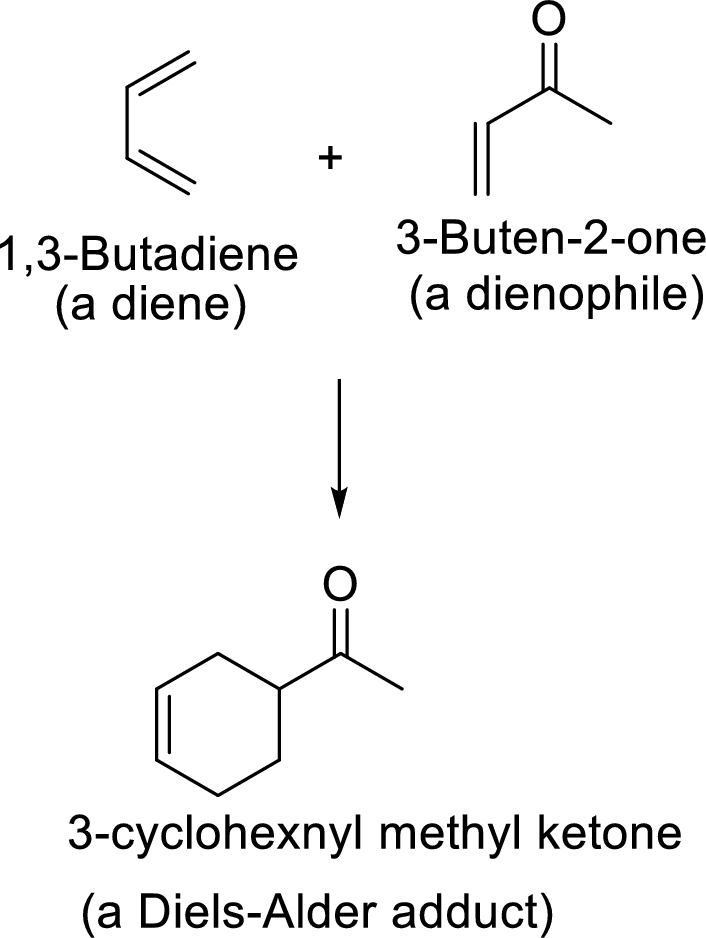
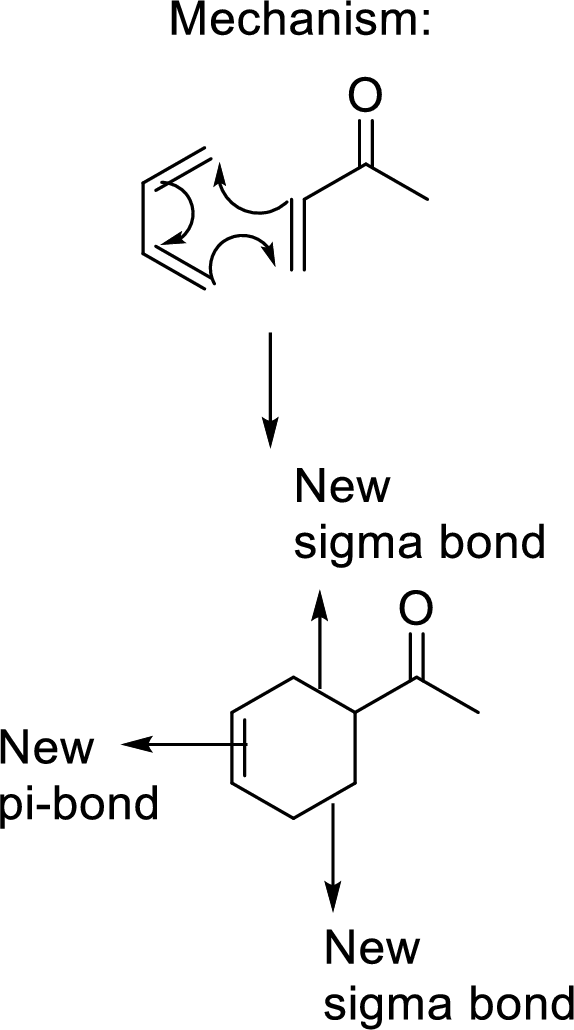
This mechanism shown that three
Trending nowThis is a popular solution!

Chapter 20 Solutions
OWL V2 with MindTap Reader and Student Solutions Manual eBook for Brown/Iverson/Anslyn/Foote's Organic Chemistry, 8th Edition
- Zingiberene and β-sesquiphellandrene, natural products obtained from ginger root, contain conjugated diene units. Which diene reacts faster in the Diels–Alder reaction and why?arrow_forwardAs many as 18 different Diels–Alder products can be obtained by heating a mixture of 1,3-butadiene and 2-methyl-1,3-butadiene. Identify the productsarrow_forwardThere is TWO Blanks, what should I put in them?arrow_forward
- True or False: Acetylene is a naturally occurring conjugated diene True or False: The Diels-Alder reaction has the stereochemistry of the dienophile is retained in the product. True or False: When looking at kinetic vs. thermodynamic products the kinetic product predominates at low temperature. True or False: the mechanism of the Diels-Alder reaction is three π bonds break; one σ bond and two π bonds form.arrow_forwardIn the Diels Alder reaction what is Kinetically controlled product and what isthermodynamically controlled product? Give reasons.arrow_forwardThe following diene does not undergo a Diels-Alder reaction with maleic anhydride, both in thermal and photochemical conditions. Explain why this reaction does not occur.arrow_forward
- What is the structure of the Diels Alder product according to this reaction?arrow_forwardDraw and discuss the mechanism (with arrows to show electron movements) of the Diels-Alder reaction between anthracene and maleic anhydride. Draw the orientation and phases of the reacting p-orbitals showing how they overlap in a “suprafacial” geometry to form productarrow_forwardWhy is the diels alder reaction important? What is a compound that would be not possible to synthesize without it?arrow_forward
- Exp3: Diels-Alder reaction Why does cyclopentadiene regularly form a dimer? How to break the dimer into monomers? Does the diene in this reaction act as the nucleophile or the electrophile? Is the maleic anhydride a nucleophile or an electrophile in this reaction? Explain your reasoning.arrow_forward[Review Topics] [References] Draw structural formulas for the diene and dienophile that combine in a Diels-Alder reaction to form the product shown. Diene + Dienophile CH3O CH₂ I wwwwwwwarrow_forwardOf the three 1,4-diphenyl-1,3-butadiene isomers (E,E or E,Z or Z,Z) indicate the most suitable diene that can be used as a reactant in a Diels-Alder reaction. Explain your choice.arrow_forward
