
Concept explainers
How would you use NMR (either 13C or 1H) to distinguish between the following pairs of isomers?
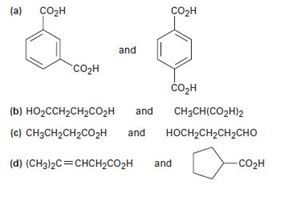
a)
Interpretation:
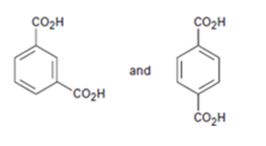
How NMR (either 13C or 1H) could be used to distinguish between benzene-1,2-dicarboxylic acid and benzene-1,3-dicarboxylic acid is to be stated.
Concept introduction:
In 1HNMR, the acidic COOH proton normally absorbs as singlet, broadened in few cases, near 12 δ.
In 13CNMR, the carboxyl carbon absorb in the range 165-185 δ. The aromatic and α, β-unsaturated acids absorb near 165 δ and saturated aliphatic acids near 185 δ. The nitrile carbons absorb in the range 115-130 δ.
Compounds with symmetrical structures (like p-disubstituted phenyl) will have a simpler spectrum.
To state:
How NMR (either 13C or 1H) could be used to distinguish between benzene-1,3-dicarboxylic acid and benzene-1,4-dicarboxylic acid.
Answer to Problem 55AP
1,4-Benzenedicarboxylic acid will have a simpler spectrum. In 1H NMR, there will be a doublet of doublet in the aromatic region and a singlet for carboxyl proton around 12 δ. In 13C NMR there will be three signals, two in aromatic region and one for carboxyl carbon around 165 δ.
The 1H NMR of 1,3-Benzenedicarboxylic acid will have a multiplet in the aromatic region due to the ring protons and a singlet for carboxyl proton around 12 δ. In 13C NMR there will be five signals, four in aromatic region and one for carboxyl carbon around 165 δ.
Explanation of Solution
1,4-Benzenedicarboxylic acid has a symmetrical structure. Hence it gives minimum peaks in both 1H NMR and 13C NMR spectrum.
In 1H NMR, the four aromatic hydrogens belong to two groups and thus give a doublet of doublet in the aromatic region. The carboxyl proton gives another signal. Thus there will be three signals.
In 13C NMR, the six ring carbons classify themselves into two groups and give two signals while the carboxyl carbons give a signal. Thus there will be three signals.
1,3-Benzenedicarboxylic acid does not have a symmetrical structure. Hence it gives more peaks in both 1H NMR and 13C NMR spectrum than 1,4-Benzenedicarboxylic acid.
In 1H NMR, the four aromatic hydrogens give complex multiplet signals in the aromatic region. The carboxyl protons yield another signal. Thus there will be two signals.
In 13C NMR, the six ring carbons classify themselves into four groups and give four signals while the carboxyl carbons give a signal. Thus there will be five signals.
1,4-Benzenedicarboxylic acid will have a simpler spectrum. In 1H NMR, there will be a doublet of doublet in the aromatic region and a singlet for carboxyl proton around 12 δ. In 13C NMR there will be three signals, two in aromatic region and one for carboxyl carbon around 165 δ.
The 1H NMR of 1,3-Benzenedicarboxylic acid will have a multiplet in the aromatic region due to the ring protons and a singlet for carboxyl proton around 12 δ. In 13C NMR there will be five signals, four in aromatic region and one for carboxyl carbon around 165 δ.
b) HO2CCH2CH2CO2H and CH3CH(CO2H)2
Interpretation:
How NMR (either 13C or 1H) could be used to distinguish between succinic acid and ethane-1,1-dicarboxylic acid is to be stated.
Concept introduction:
In 1HNMR, the acidic COOH proton normally absorbs as singlet, broadened in few cases, near 12 δ.
In 13CNMR, the carboxyl carbon absorb in the range 165-185 δ. The aromatic and α, β-unsaturated acids absorb near 165 δ and saturated aliphatic acids near 185 δ. The nitrile carbons absorb in the range 115-130 δ.
Compounds with symmetrical structures (like p-disubstituted phenyl) will have a simpler spectrum.
To state:
How NMR (either 13C or 1H) could be used to distinguish between succinic acid and ethane-1,1-dicarboxylic acid.
Answer to Problem 55AP
Succinic acid will have a simpler spectrum. In 1H NMR, there will be two signals, a triplet for the methylene groups and a singlet for carboxyl protons around 12 δ. In 13C NMR there will be two signals, one for the methylene carbon and one for carboxyl carbons around 165 δ.
The 1H NMR, 2-methylmalonic acid will have three signals, a doublet for the methyl protons, a quartet for the methane proton and a singlet for carboxyl proton around 12 δ. In 13C NMR there will be three signals, one for methyl carbon another for the methine carbon and a third one for carboxyl carbons around 165 δ.
Explanation of Solution
Succinic acid has a simpler spectrum since it is symmetrical. In 1H NMR, the two methylene protons split each other giving a triplet integrating to four protons. The two carboxyl protons yield another singlet signal. Thus there will be two signals a triplet and a singlet.
In 13C NMR, the four carbons in succinic acid classify themselves into two groups and give two signals. Thus there will be two signals, one for methylene carbons and other for carboxyl carbons.
2-methylmalonicacid does not have a symmetrical structure. Hence it gives more peaks in both 1H NMR and 13C NMR spectrum than succinic acid.
In 1H NMR, there will be a doublet for the methyl protons, a quartet integrating to one proton for the methine proton signal and a singlet for the carboxyl protons. Thus there will be three signals.
In 13C NMR, there will be separate signals for methyl carbon, methine carbon and carboxyl carbons. Thus there will be three signals.
Succinic acid will have a simpler spectrum. In 1H NMR, there will be two signals, a triplet for the methylene groups and a singlet for carboxyl protons around 12 δ. In 13C NMR there will be two signals, one for the methylene carbons and one for carboxyl carbons around 165 δ.
The 1H NMR, 2-methylmalonic acid will have three signals, a doublet for the methyl protons, a quartet for the methine proton and a singlet for carboxyl proton around 12 δ. In 13C NMR there will be three signals, one for methyl carbon another for the methine carbon and a third one for carboxyl carbons around 165 δ.
c) CH3CH2CH2CO2H and HOCH2CH2CH2CHO
Interpretation:
How NMR (either 13C or 1H) could be used to distinguish between succinic acid and 4-hydroxybutanal is to be stated.
Concept introduction:
In 1HNMR, the acidic COOH proton normally absorbs as singlet, broadened in few cases, near 12 δ.
In 13CNMR, the carboxyl carbon absorb in the range 165-185 δ. The aromatic and α, β-unsaturated acids absorb near 165 δ and saturated aliphatic acids near 185 δ. The nitrile carbons absorb in the range 115-130 δ.
Compounds with symmetrical structures will have a simpler spectrum.
In 1HNMR the aldehyde protons absorb near 10 δ with a coupling constant, J=3Hz. Hydrogens on the carbon next to aldehyde group absorb near 2.0-2.3 δ.
In 13CNMR, saturated aldehydes and ketones usually absorb in the region from 200 to 215 δ while the aromatic and unsaturated carbonyl compounds absorb in the 190 to 200 δ region.
To state:
How NMR (either 13C or 1H) could be used to distinguish between butanoic acid and 4-hydroxybutanal.
Answer to Problem 55AP
In 1H NMR of butanoic acid there will be a signal around 12 δ while 1H NMR of 3-hydroxybutanal there will be a signal for the aldehydic proton around 9-10 δ and for the hydroxyl proton in the range 3.0-4.5 δ. There will be no signal around 12 δ. In 13C NMR the carboxyl carbon absorbs around 165 δ while the absorption due to aldehydic carbon is observed around 200-215 δ.
Explanation of Solution
The two compounds can be easily distinguished. For butanoic acid there will be signals for the absorption of carboxyl proton in 1H NMR (12 δ) and carboxyl carbon in 13C NMR (165 δ). In 3-hydroxybutanal, there will be signals for the absorption of hydroxyl proton in 1H NMR (3.0-4.5 δ) and aldehydic proton around 9-10 δ. The aldehydic carbon will resonate in 13C NMR in the range 200-215 δ.
In 1H NMR of butanoic acid there will be a signal around 12 δ while 1H NMR of 3-hydroxybutanal there will be a signal for the aldehydic proton around 9-10 δ and for the hydroxyl proton in the range 3.0-4.5 δ. There will be no signal around 12 δ. In 13C NMR the carboxyl carbon absorbs around 165 δ while the absorptrion due to aldehydic carbon is observed around 200-215 δ.
d)

Interpretation:
How NMR (either 13C or 1H) could be used to distinguish between 4-methylpent-3-eneoic acid and cyclopentylcarboxylic acid is to be stated.
Concept introduction:
In 1HNMR, the acidic COOH proton normally absorbs as singlet, broadened in few cases, near 12 δ.
In 13CNMR, the carboxyl carbon absorb in the range 165-185 δ. The aromatic and α, β-unsaturated acids absorb near 165 δ and saturated aliphatic acids near 185 δ. The nitrile carbons absorb in the range 115-130 δ.
Alkenes show a signal in the range 4.5-6.5 δ in 1HNMR and around 110-150 δ in 13CNMR.
Compounds with symmetrical structures will have a simpler spectrum.
To state:
How NMR (either 13C or 1H) could be used to distinguish between 4-methylpent-3-eneoic acid and cyclopentylcarboxylic acid.
Answer to Problem 55AP
In 1HNMR, 4-methylpent-3-eneoic acid will show the peak due to olefinic proton absorption in the range 4.5-6.5 δ. In 13CNMR, the two carbons in the double bond each will give a signal in the range 110-150 δ. There will be no signals in these ranges for cyclopentanecarboxylic acid in both the spectra.
Explanation of Solution
4-Methylpent-3-eneoic acid has a hydrogen atom attached to olefinic double bond. Since it is a vinylic hydrogen its absorption peak is observed in the range 4.5-6.5 δ. The two olefinic carbons are not equivalent. Hence they give two signals in the range 110-150 δ. In the 1HNMR and 13CNMR spectra of cyclopentanecarboxylic acid no signals will be observed in the ranges 4.5-6.5 δ and 110-150 δ respectively.
In 1HNMR, 4-methylpent-3-eneoic acid will show the peak due to olefinic proton absorption in the range 4.5-6.5 δ. In 13CNMR, the two carbons in the double bond each will give a signal in the range 110-150 δ. There will be no signals in these ranges for cyclopentanecarboxylic acid in both the spectra.
Want to see more full solutions like this?
Chapter 20 Solutions
Bundle: Organic Chemistry, Loose-leaf Version, 9th + LMS Integrated for OWLv2, 4 terms (24 months) Printed Access Card
- Identify the structures of isomers A and B, whose molecular formula is C10 H12 O2, from IR spectroscopic data and their H-NMR spectra.arrow_forwardCompound A, C8H10O, has the IR and 1H NMR spectra shown. Propose a structure consistent with the observed spectra, and label each peak in the NMR spectrum. Note that the absorption at 5.5 î disappears when D2O is added.arrow_forwardIdentify the following molecules based on their IR, 13C and 1N NMR spectraarrow_forward
- Compound C has molecular formula C5H8O. The IR, mass, 1H-NMR, and 13C-NMR spectra are shown below. Suggest a structure for C and explain your reasoning.arrow_forwardPropose a structure for each of the following two isomers with formula C6H14 given their 1H-NMR spectra. Isomer A: δ = 0.84 (d, 12 H), 1.39 (septet, 2H) ppm Isomer B: δ = 0.84 (t, 3 H), 0.86 (t, 9H), 1.22 (q, 2H) ppmarrow_forwardHow could 1H NMR spectroscopy be used to distinguish among isomers A, B, and C?arrow_forward
- Compound C shows a molecular ion at m/z 148 and other prominent peaks at m/z 105 and 77. Following are its infrared and 1H-NMR spectra. Q.) Deduce the structural formula of compound Carrow_forwardCompound I (C11H14O2) is insoluble in water, aqueous acid, and aqueous NaHCO3, but dissolves readily in 10% Na2CO3 and 10% NaOH. When these alkaline solutions are acidified with 10% HCl, compound I is recovered unchanged. Given this information and its 1H-NMR spectrum, deduce the structure of compound I.arrow_forward3-Chlorocyclopropene, on treatment with AgBF4, gives a precipitate of AgCl and a stable solution of a product that shows a single 1H NMR absorption at 11.04 δ. What is a likely structure for the products, and what is its relation to HĂ¼ckel’s rule?arrow_forward
- 1, 6-Methanonaphthalene has an interesting 1H NMR spectrum in which the eight hydrogens around the perimeter absorb at 6.9 to 7.3 δ, while the two CH2 protons absorb at -0.5 δ. Tell whether it is aromatic, and explain its NMR spectrum.arrow_forwardPropose a structural formula for the analgesic phenacetin, molecular formula C10H13NO2, based on its 1H-NMR spectrum.arrow_forwardThree isomeric compounds, A, B, and C, all have molecular formulaC8H11N. The 1H NMR and IR spectral data of A, B, and C are given below.What are their structures?arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

