
Concept explainers
(a)
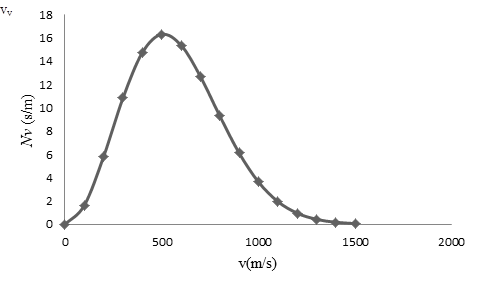
To draw: The graph of the Maxwell speed distribution function versus speed with points at speed intervals of
(a)
Answer to Problem 62AP
The of the Maxwell speed distribution function versus speed with points at speed intervals of
Explanation of Solution
Introduction:
The Maxwell distribution curve is the graph between the distribution of speed and the change in speed or speed interval.
Given info: The number of molecules of oxygen in vessel is
Write the expression of Maxwell’s speed distribution function.
Here,
The mass of the molecules of oxygen is,
Here,
The molecular mass of the oxygen molecules in
Substitute
Substitute
Substitute the values of
|
|
|
| 0 | 0 |
| 100 | 1.64 |
| 200 | 5.86 |
| 300 | 10.88 |
| 400 | 14.78 |
| 500 | 16.33 |
| 600 | 15.39 |
| 700 | 12.7 |
| 800 | 9.31 |
| 900 | 6.13 |
| 1000 | 3.64 |
| 1100 | 1.961 |
| 1200 | 0.96 |
| 1300 | 0.43 |
| 1400 | 0.18 |
| 1500 | 0.07 |
On the basis of the table, a graph is plotted below;
(b)
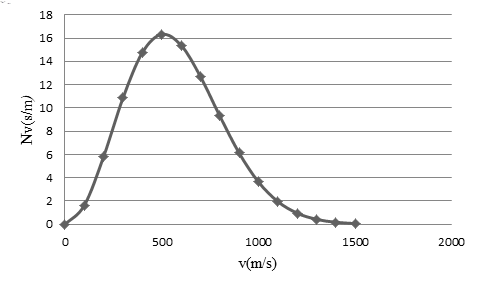
The most probable speed from the graph.
(b)
Answer to Problem 62AP
The most probable speed is
Explanation of Solution
Given info: The number of molecules of oxygen in vessel is
The most probable speed occurs where
Conclusion:
Therefore, the most probable speed is
(c)
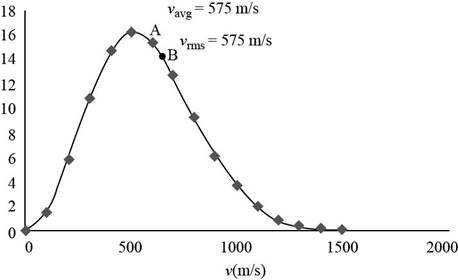
The average and rms speeds for the molecules and label these points on the graph.
(c)
Answer to Problem 62AP
The average and rms speeds for the molecules is
Explanation of Solution
Given info: The number of molecules of oxygen in vessel is
Write the expression of average velocity.
The mass of the molecules of oxygen is,
Substitute
The molecular mass of the oxygen molecules in
Substitute
Thus, the average speed is
Write the expression of rms velocity.
Substitute
Substitute
Thus, the rms velocity of the oxygen molecules is
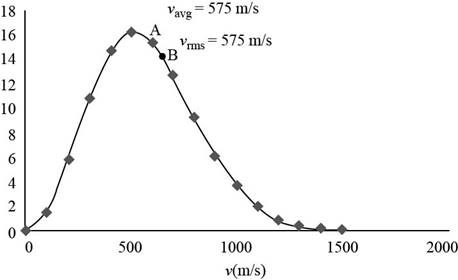
The graph of Maxwell’s curve is shown below;
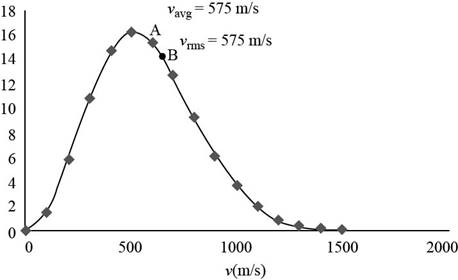
Figure (1)
The point
Conclusion:
Therefore, the average and rms speeds for the molecules is
(d)
The fraction of molecules with the speed in the range of
(d)
Answer to Problem 62AP
The fraction of molecules with the speed in the range of
Explanation of Solution
Given info: The number of molecules of oxygen in vessel is
The figure given below shows the Maxwell’s curve,
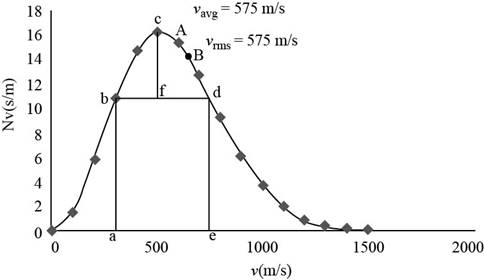
Figure 2
Write the expression of the fraction of molecules with the speed range from
Substitute
Conclusion:
Therefore, the fraction of molecules with the speed in the range of
Want to see more full solutions like this?
Chapter 21 Solutions
Physics For Scientists And Engineers With Modern Physics, 9th Edition, The Ohio State University
- From the MaxwellBoltzmann speed distribution, show that the most probable speed of a gas molecule is given by Equation 16.23. Note: The most probable speed corresponds to the point at which the slope of the speed distribution curve dNv/dv is zero.arrow_forwardFind (a) the most probable speed, (b) the average speed, and (c) the rms speed for nitrogen molecules at 295 K.arrow_forwardCylinder A contains oxygen (O2) gas, and cylinder B contains nitrogen (N2) gas. If the molecules in the two cylinders have the same rms speeds, which of the following statements is false? (a) The two gases haw different temperatures. (b) The temperature of cylinder B is less than the temperature of cylinder A. (c) The temperature of cylinder B is greater than the temperature of cylinder A. (d) The average kinetic energy of the nitrogen molecules is less than the average kinetic energy of the oxygen molecules.arrow_forward
- An ideal gas is contained in a vessel at 300 K. The temperature of the gas is then increased to 900 K. (i) By what factor does the average kinetic energy of the molecules change, (a) a factor of 9, (b) a factor of 3, (c) a factor of 3, (d) a factor of 1, or (e) a factor of 13? Using the same choices as in part (i), by what factor does each of the following change: (ii) the rms molecular speed of the molecules, (iii) the average momentum change that one molecule undergoes in a collision with one particular wall, (iv) the rate of collisions of molecules with walls, and (v) the pressure of the gas?arrow_forwardOne cylinder contains helium gas and another contains krypton gas at the same temperature. Mark each of these statements true, false, or impossible to determine from the given information. (a) The rms speeds of atoms in the two gases are the same. (b) The average kinetic energies of atoms in the two gases are the same. (c) The internal energies of 1 mole of gas in each cylinder are the same. (d) The pressures in the two cylinders ale the same.arrow_forwardA sample of a monatomic ideal gas occupies 5.00 L at atmospheric pressure and 300 K (point A in Fig. P17.68). It is warmed at constant volume to 3.00 atm (point B). Then it is allowed to expand isothermally to 1.00 atm (point C) and at last compressed isobarically to its original state. (a) Find the number of moles in the sample. Find (b) the temperature at point B, (c) the temperature at point C, and (d) the volume at point C. (e) Now consider the processes A B, B C, and C A. Describe how to carry out each process experimentally. (f) Find Q, W, and Eint for each of the processes. (g) For the whole cycle A B C A, find Q, W, and Eint. Figure P17.68arrow_forward
- Fifteen identical particles have various speeds: one has a speed of 2.00 m/s, two have speeds of 3.00 m/s, three have speeds of 5.00 m/s, four have speeds of 7.00 m/s, three have speeds of 9.00 m/s, and two have speeds of 12.0 m/s. Find (a) the average speed, (b) the rms speed, and (c) the most probable speed of these particles.arrow_forwardEight bumper cars, each with a mass of 322 kg. are running in a room 21.0 m long and 130 m wide. They have no driver, so they just bounce around on their own. The rms speed of the cars is 2.50 m/s. Repeating the arguments of Pressure, Temperature, and RMS Speed, find the average force per unit length (analogous to Pressure) that the cars exert the walls.arrow_forwardOn a hot summer day, the density of air at atmospheric pressure at 35.0C is 1.1455 kg/m3. a. What is the number of moles contained in 1.00 m3 of an ideal gas at this temperature and pressure? b. Avogadros number of air molecules has a mass of 2.85 102 kg. What is the mass of 1.00 m3 of air? c. Does the value calculated in part (b) agree with the stated density of air at this temperature?arrow_forward
- A sample of a monatomic ideal gas occupies 5.00 L at atmospheric pressure and 300 K (point A in Fig. P21.65). It is warmed at constant volume to 3.00 atm (point B). Then it is allowed to expand isothermally to 1.00 atm (point C) and at last compressed isobarically to its original state, (a) Find the number of moles in the sample. Find (b) the temperature at point B, (c) the temperature at point C, and (d) the volume at point C. (e) Now consider the processes A B, B C, and C A. Describe how to carry out each process experimentally, (f) Find Q, W, and Eint for each of the processes, (g) For the whole cycle A B C A, find Q, W, and Eint.arrow_forwardOne process for decaffeinating coffee uses carbon dioxide ( M=44.0 g/mol) at a molar density of about 14,0 mol/m3 and a temperature of about 60 . (a) Is CO2 a solid, liquid, gas, or supercritical fluid under those conditions? (b) The van der Waals constants for carbon dioxide are a=0.3658 Pa m6/mol2 and b=4.286105 m3/mol. Using the van der Waals equation, estimate pressure of CO2 at that temperature and density. `arrow_forwardAn aluminum rod 0.500 m in length and with a cross-sectional area of 2.50 cm2 is inserted into a thermally insulated vessel containing liquid helium at 4.20 K. The rod is initially at 300 K. (a) If one-half of the rod is inserted into the helium, how many liters of helium boil off by the time the inserted half cools to 4.20 K? Assume the upper half does not yet cool. (b) If the circular surface of the upper end of the rod is maintained at 300 K, what is the approximate boil-off rate of liquid helium in liters per second after the lower half has reached 4.20 K? (Aluminum has thermal conductivity of 3 100 W/m K at 4.20 K; ignore its temperature variation. The density of liquid helium is 125 kg/m3.)arrow_forward
 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning





