
Concept explainers
(a)
Interpretation:
The type of isomerism that could be exhibited by the given formulae and an example that illustrates the specific type of isomerism has to be stated.
Concept introduction:
Organic compounds which have similar chemical formula but different structures, i.e., the atoms are in a different spatial arrangement, they are known as structural isomers. Cis and Trans isomers are also structural or geometrical isomers.
(a)
Answer to Problem 2Q
Explanation of Solution
The type of isomerism that could be exhibited by
The structural isomer of
The given formula,
The root word hexene indicates that six carbon atoms are present in the parent chain. Double bond is present between first and second carbon.
The structure of 1-hexene is shown
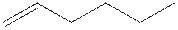
The structural isomer of
The given formula,
The root word hexene indicates that six carbon atoms are present in the parent chain. The number
The structure of 2-hexene is shown
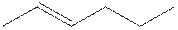
The structural isomer of
The given formula,
The root word hexene indicates that six carbon atoms are present in the parent chain. The number
The structure of 3-hexene is shown
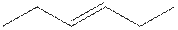
The structural isomer of
The given formula,
The root word pentene indicates that five carbon atoms are present in the parent chain. The number
The structure of

The structural isomer of
The given formula,
The root word butene indicates that six carbon atoms are present in the parent chain. The double bond is present between second and third carbon. Two methyl groups are attached at second and third carbon.
The structure of
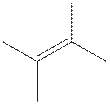
The geometric isomer of
The structure of cis-2-hexene is,
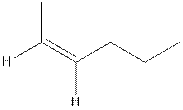
The structure of trans-2-hexene is,
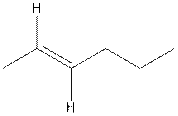
In the cis isomer, the two hydrogen of the double bonded carbons are adjacent to each other while in trans isomer, they are opposite to each other.
The geometric isomer of
The structure of cis-3-hexene is,
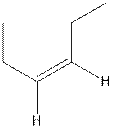
The structure of trans-3-hexene is,
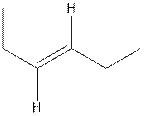
In the cis isomer, the two hydrogen of the double bonded carbons are adjacent to each other while in trans isomer, they are opposite to each other.
(b)
Interpretation:
The type of isomerism that could be exhibited by the given formulae and an example that illustrates the specific type of isomerism has to be stated.
Concept introduction:
Organic compounds which have similar chemical formula but different structures, i.e., the atoms are in a different spatial arrangement, they are known as structural isomers. Cis and Trans isomers are also structural or geometrical isomers.
(b)
Answer to Problem 2Q
Explanation of Solution
The type of isomerism that could be exhibited by
The structural isomer of
The root word pentane indicates that five carbon atoms are present in the parent chain. Hydroxyl group is attached to first carbon.
The structure of pentan-1-ol is shown,

The structural isomer of
The root word butane indicates that four carbon atoms are present in the parent chain.
The structure of
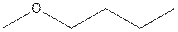
The structural isomer of
The root word propane indicates that three carbon atoms are present in the parent chain.
The structure of 2-ethoxypropane is shown

The structural isomer of
The root word butane indicates that four carbon atoms are present in the parent chain.
The structure of 2-methoxybutane is shown
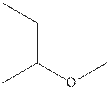
The structural isomer of
The root word pentane indicates that five carbon atoms are present in the parent chain. Hydroxyl group is attached to third carbon.
The structure of pentan-
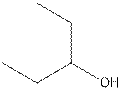
The structural isomer of
The root word pentane indicates that five carbon atoms are present in the parent chain. Hydroxyl group is attached to second carbon.
The structure of pentan-

(c)
Interpretation:
The type of isomerism that could be exhibited by the given formulae and an example that illustrates the specific type of isomerism has to be stated.
Concept introduction:
Organic compounds which have similar chemical formula but different structures, i.e., the atoms are in a different spatial arrangement, they are known as structural isomers. Cis and Trans isomers are also structural or geometrical isomers.
(c)
Answer to Problem 2Q
Explanation of Solution
The type of isomerism that could be exhibited by
The structural isomer of
In
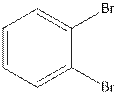
The structural isomer of
In
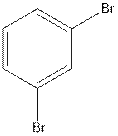
The structural isomer of
In
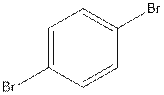
Want to see more full solutions like this?
Chapter 22 Solutions
Chemistry: Cengage Technology Edition
- given the following organic compounds, identify and explain the types of isomers in those samplesarrow_forwarda. How many structural isomers exist for C4H9OH? b. (i) Write condensed structural formulas, and sketch skeletal structures for each structural isomer of C4H9OH. (ii) How many structural isomer(s) is / are chiral?arrow_forward
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





