
EBK ORGANIC CHEMISTRY
12th Edition
ISBN: 9781119233664
Author: Snyder
Publisher: VST
expand_more
expand_more
format_list_bulleted
Question
Chapter 24, Problem 20P
Interpretation Introduction
Interpretation:
Outlining the reactions that are involved in the synthesis of DL-Glutamic acid from diethyl acetamidomalonate. Outlining the reactions which are involved in the preparation of DL-ornithine by the intermediate compound, ‘G’.
Concept Introduction:
The reaction of an
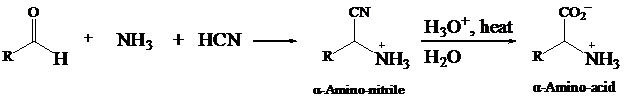
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
(a) Compound Z is a tertiary aromatic amine with the formula, C8H11N. Provide a chemical structure for compound Z.
(b)nDraw the structure of the product formed exclusively when nitrous acid reacts with Z.
Please draw the skeletal formula of the following compounds:
(A) Isobutyraldehyde
(B) α-Ethyl-γ-methoxycaproaldehyde
(C) 6-Hydroxyhexanal
(D) 2,4-Pentanedione
(E)3-Cyano-7-oxoheptanoic acid
(b)
(i)
The reaction between aromatic compound W, C,H$OC1 with an amine X, CH>N was
carried out. The compound Y was extracted and purified from this reaction. It was
then reduced by LİAIH4 followed by hydrolysis to obtain compound Z, which is a
tertiary amine. Draw the structures for compounds W, X, Y and Z.
Chapter 24 Solutions
EBK ORGANIC CHEMISTRY
Ch. 24 - Prob. 1PPCh. 24 - Practice Problem 24.2 The guanidino group NHNHCNH2...Ch. 24 - Prob. 3PPCh. 24 - Prob. 4PPCh. 24 - Prob. 5PPCh. 24 - Prob. 6PPCh. 24 - Prob. 7PPCh. 24 - Practice Problem 24.8
Glutathione is a tripeptide...Ch. 24 - Prob. 9PPCh. 24 - Prob. 10PP
Ch. 24 - Prob. 11PPCh. 24 - Practice Problem 24.12 Show all steps in the...Ch. 24 - Practice Problem 24.13 The synthesis of a...Ch. 24 - Practice Problem 24.14
The terminal carboxyl...Ch. 24 - Prob. 15PPCh. 24 - Prob. 16PPCh. 24 - (a) Which amino acids in Table 24.1 have more than...Ch. 24 - Prob. 18PCh. 24 - 24.19 (a) On the basis of the following sequence...Ch. 24 - Prob. 20PCh. 24 - Prob. 21PCh. 24 - Prob. 22PCh. 24 - Prob. 23PCh. 24 - Prob. 24PCh. 24 - The enzyme lysozyme and its mechanism are...Ch. 24 - Prob. 2LGP
Knowledge Booster
Similar questions
- Predict the products formed in each of the following reactions, and write a balanced equation: (a) CH3NHNH2(g) + O2(8) → ? (b) Mg3P2(s) + H20(1) → ?arrow_forwardα-Amino acids can be prepared by treating an aldehyde with ammonia/trace acid, followed by hydrogen cyanide, followed by acid-catalyzed hydrolysis. Draw the structures of the two intermediates formed in this reaction.arrow_forwardGiven that C6H11COOH has a pKa = 4.8 and C6H11N+H3 has a pKa = 10.7, (a) What pH would you make the water layer to cause the carboxylic acid to dissolve in the water layer and the amine to dissolve in the ether layer? (b) What pH would you make the water layer to cause the carboxylic acid to dissolve in the ether layer and the amine to dissolve in the water layer?arrow_forward
- (a) Draw the three isomers of benzenedicarboxylic acid.(b) The isomers have melting points of 210 °C, 343 °C, and 427 °C. Nitration of the isomers at all possible positions was once used to determine their structures. The isomer that melts at 210 °C gives two mononitro isomers. The isomer that melts at 343 °C gives three mononitro isomers. The isomer that melts at 427 °C gives only one mononitro isomer. Show which isomer has which melting point.arrow_forwardSynthesize attached compound from benzonitrile (C6H5CN) as the onlyorganic starting material; that is, every carbon in the product mustoriginate in benzonitrilearrow_forwardUsing the data in Appendix C, determine which of the following bases is strong enough to deprotonate acetonitrile (CH3CN), so that equilibrium favors the products: (a) NaH; (b) Na2CO3; (c) NaOH; (d) NaNH2; (e) NaHCO3.arrow_forward
- Ciprofloxacin is a member of the fluoroquinolone class of antibiotics.(a) Which of its rings are aromatic?arrow_forwardWhen the conjugate acid of aniline, C6H5NH3+, reacts with the acetate ion, the following reaction takes place: C6H5NH3+(aq)+CH3COO(aq)C6H5NH2(aq)+CH3COOH(aq) If Kafor C6H5NH3+ is 1.35105 and Kafor CH3COOH is 1.86105 , what is K for the reaction?arrow_forwardAspirin, or 2-acetoxybenzoic acid, (C9H8O4) is often synthesised from salicylic acid.(i) Sketch and discuss any changes in the number of possible structural conformations ofaspirin relative to those of salicylic acid. (ii) Re-draw the structure predicted to be the lowest energy conformation of aspirin,indicating any expected stabilising and destabilising interactions. Justify your choice.arrow_forward
- Indicate whether each statement is true or false: (a) Fat molecules contain amide bonds. (b) Phosphoplipids can be zwitterions. (c) Phospholipids form bilayers in water in order to have their long hydrophobic tails interact favorably with each other, leaving their polar heads to the aqueous environment.arrow_forwardDraw the structural formulas of the following compounds:(a) 2,3-Dimethylpentanal(b) 1,3-Dibromopropanone(c) 4-hydroxy-4-methylhexan-2-onearrow_forwardGive suggestion the structural formula for the following compounds. a) m-aminobenzoic acidb) 4-benzyl-2,7-dimethyl-8-phenyloctenearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning