
FUND.OF GEN CHEM CHAP 1-13 W/ACCESS
16th Edition
ISBN: 9781323406038
Author: McMurry
Publisher: PEARSON C
expand_more
expand_more
format_list_bulleted
Question
Chapter 2.4, Problem 2.8P
Interpretation Introduction
Interpretation:
The given metalloids should be identified in the periodic table.
Concept introduction:
The periodic table is given below,
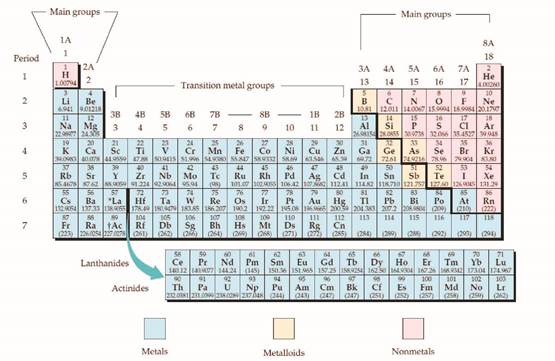
Figure 1
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Another major use of bismuth has been as an ingredient in low-melting metal alloys, such as those used in firesprinkler systems and in typesetting. The element itself is a brittle white crystalline solid. How do these characteristicsfit with the fact that bismuth is in the same periodic group with such nonmetallic elements as nitrogenand phosphorus?
What is the empirical formula for a compound that is 26.56% potassium, 35.41% chromium, and 38.03% oxygen?
What is the empirical formula of a compound that contains 72.0% carbon, 12.0% hydrogen and 16.0% oxygen by mass?
Chapter 2 Solutions
FUND.OF GEN CHEM CHAP 1-13 W/ACCESS
Ch. 2.1 - Prob. 2.1CIAPCh. 2.1 - For the Kanji character in the lower portion of...Ch. 2.2 - Use the list inside the front cover to identify...Ch. 2.3 - Prob. 2.2PCh. 2.3 - Prob. 2.3PCh. 2.3 - Prob. 2.4PCh. 2.4 - Prob. 2.5PCh. 2.4 - Prob. 2.6PCh. 2.4 - Prob. 2.7PCh. 2.4 - Prob. 2.8P
Ch. 2.4 - Prob. 2.9PCh. 2.5 - Prob. 2.10PCh. 2.5 - Prob. 2.11PCh. 2.5 - Prob. 2.12PCh. 2.5 - Prob. 2.13KCPCh. 2.5 - Prob. 2.3CIAPCh. 2.5 - Prob. 2.4CIAPCh. 2.6 - Prob. 2.14PCh. 2.7 - Prob. 2.15PCh. 2.7 - Write electron configurations for the following...Ch. 2.7 - Prob. 2.17PCh. 2.7 - Identify the atom with the following...Ch. 2.8 - Prob. 2.19PCh. 2.8 - Prob. 2.20PCh. 2.8 - Prob. 2.21PCh. 2.8 - Prob. 2.22KCPCh. 2.9 - Prob. 2.23PCh. 2.9 - Write electron-dot symbols for radon, lead, xenon,...Ch. 2.9 - Prob. 2.25PCh. 2.9 - Prob. 2.5CIAPCh. 2.9 - Prob. 2.6CIAPCh. 2 - Where on the following outline of a periodic table...Ch. 2 - Is the element marked in red on the following...Ch. 2 - Prob. 2.28UKCCh. 2 - What atom has the following orbital-filling...Ch. 2 - Use the following orbital-filling diagram to show...Ch. 2 - What four fundamental assumptions about atoms and...Ch. 2 - How do atoms of different elements differ?Ch. 2 - Prob. 2.33APCh. 2 - Prob. 2.34APCh. 2 - Prob. 2.35APCh. 2 - Prob. 2.36APCh. 2 - How many O atoms of mass 15.99 amu are in 15.99 g...Ch. 2 - Prob. 2.38APCh. 2 - What are the names of the three subatomic...Ch. 2 - Prob. 2.40APCh. 2 - Prob. 2.41APCh. 2 - Prob. 2.42APCh. 2 - Which of the following symbols represent isotopes...Ch. 2 - Prob. 2.44APCh. 2 - Prob. 2.45APCh. 2 - Prob. 2.46APCh. 2 - One of the most widely used isotopes in medical...Ch. 2 - Prob. 2.48APCh. 2 - Prob. 2.49APCh. 2 - Prob. 2.50APCh. 2 - Prob. 2.51APCh. 2 - Prob. 2.52APCh. 2 - Prob. 2.53APCh. 2 - Prob. 2.54APCh. 2 - Prob. 2.55APCh. 2 - For (a) rubidium (b) tungsten, (c) germanium, and...Ch. 2 - For (a) calcium, (b) palladium, (c) carbon, and...Ch. 2 - Prob. 2.58APCh. 2 - Prob. 2.59APCh. 2 - Prob. 2.60APCh. 2 - Prob. 2.61APCh. 2 - Prob. 2.62APCh. 2 - Prob. 2.63APCh. 2 - Prob. 2.64APCh. 2 - Prob. 2.65APCh. 2 - Prob. 2.66APCh. 2 - Prob. 2.67APCh. 2 - Prob. 2.68APCh. 2 - Prob. 2.69APCh. 2 - Prob. 2.70APCh. 2 - Prob. 2.71APCh. 2 - Determine the number of unpaired electrons for...Ch. 2 - Without looking back in the text, write the...Ch. 2 - Prob. 2.74APCh. 2 - Prob. 2.75APCh. 2 - Prob. 2.76APCh. 2 - Prob. 2.77APCh. 2 - Prob. 2.78APCh. 2 - Using n for the number of the valence shell and...Ch. 2 - What elements in addition to helium make up the...Ch. 2 - Prob. 2.81CPCh. 2 - What is the atomic number of the yet-undiscovered...Ch. 2 - Give the number of electrons in each shell for...Ch. 2 - Identify the highest-energy occupied subshell in...Ch. 2 - Prob. 2.85CPCh. 2 - Prob. 2.86CPCh. 2 - Germanium, atomic number 32, is used in building...Ch. 2 - Prob. 2.88CPCh. 2 - Prob. 2.89CPCh. 2 - What is wrong with the following electron...Ch. 2 - Prob. 2.91CPCh. 2 - Prob. 2.92CPCh. 2 - Prob. 2.93CPCh. 2 - Prob. 2.94CPCh. 2 - Prob. 2.95GPCh. 2 - Prob. 2.96GPCh. 2 - Prob. 2.97GPCh. 2 - Look again at the trends illustrated in Figures...
Knowledge Booster
Similar questions
- What three things did the bohr model add to our understanding of atomic structure?arrow_forwardWhat is the mass in grams of 6.022 * 1023 O atoms of mass 16.00 amu?arrow_forwardIf one compound has the formula C5H10 and another has the formula C4H10, are the two compounds isomers? Explain.arrow_forward
- Arrange in order of increasing nonmetallic character (a) the period 3 elements Na, Cl, & Mg (b) the Group 7A elements At, F, & Iarrow_forwardElements have varying numbers of protons, neutrons, and electrons.True or false?arrow_forwardHow does the atomic structure or composition of the versions of sodium in question C above differ from a typical sodium atom, with its atomic mass of 23?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you

 Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning
Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning


Principles Of Radiographic Imaging: An Art And A ...
Health & Nutrition
ISBN:9781337711067
Author:Richard R. Carlton, Arlene M. Adler, Vesna Balac
Publisher:Cengage Learning