
ANATOMY+PHYSIOLOGY (LOOSELEAF)-W/ACCESS
6th Edition
ISBN: 9780134493992
Author: Marieb
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 25, Problem 12MC
The pH of blood varies directly with (a) HCO3–, (b) 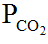 , (c) H+, (d) none of the above.
, (c) H+, (d) none of the above.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The pH of blood varies directly with (a) HCO3−, (b) PCO2, (c) H+, (d) none of the above.
The pH of human blood is buffered at 7.35 to 7.45. If the [OH-] of the blood sample is 6.3x10-6, calculate its pH value. Is the patient’s pH within the range? Or is experiencing acidosis or alkalosis condition?
What is the function of the buffer bicarbonate in the human body?
Chapter 25 Solutions
ANATOMY+PHYSIOLOGY (LOOSELEAF)-W/ACCESS
Ch. 25.1 - Which do you have more of, extracellular or...Ch. 25.1 - What is the major cation in the ECF? In ICF? What...Ch. 25.1 - If you eat salty pretzels without drinking, what...Ch. 25.2 - Prob. 6CYUCh. 25.2 - ADH, by itself, cannot reduce an increase in...Ch. 25.2 - For each of the following, state whether it might...Ch. 25.3 - Nathan has Addisons disease (insufficient...Ch. 25.3 - Prob. 9CYUCh. 25.3 - Prob. 10CYUCh. 25.4 - Define acidemia and alkalemia.
Ch. 25.4 - What are the bodys three major chemical buffer...Ch. 25.4 - Joanne, a diabetic patient, is at the emergency...Ch. 25.5 - Reabsorption of HCO3 is always tied to the...Ch. 25.5 - Prob. 14CYUCh. 25.5 - Prob. 15CYUCh. 25.5 - Prob. 16CYUCh. 25.6 - Which two abnormalities in plasma are key features...Ch. 25.6 - How do the kidneys compensate for respiratory...Ch. 25 - Body water content is greatest in (a) infants, (b)...Ch. 25 - Potassium, magnesium, and phosphate ions are the...Ch. 25 - Sodium balance is regulated primarily by control...Ch. 25 - Prob. 4MCCh. 25 - Two main substances regulated by the influence of...Ch. 25 - Two substances regulated by parathyroid hormone.Ch. 25 - Two substances secreted into the proximal...Ch. 25 - Prob. 8MCCh. 25 - Prob. 9MCCh. 25 - Prob. 10MCCh. 25 - Prob. 11MCCh. 25 - The pH of blood varies directly with (a) HCO3, (b)...Ch. 25 - In an individual with metabolic acidosis, a clue...Ch. 25 - Name the body fluid compartments, noting their...Ch. 25 - Prob. 2SAQCh. 25 - Prob. 3SAQCh. 25 - Prob. 4SAQCh. 25 - Prob. 5SAQCh. 25 - Explain how the chemical buffer systems resist...Ch. 25 - Explain the relationship of the following to renal...Ch. 25 - Mr. Heyden, a somewhat stocky 72-year-old man, is...Ch. 25 - Mr. Heyden, a somewhat stocky 72-year-old man, is...Ch. 25 - Mr. Heyden, a somewhat stocky 72-year-old man, is...Ch. 25 - Mr. Heyden, a somewhat stocky 72-year-old man, is...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- Bicarbonate buffer in blood involves the dissociation of____________________.arrow_forwardIf you hold your breath for 30 seconds, what is likely to happen to your blood pH?arrow_forwardMs Young presents to the emergency room department in respiratory distress. The respiratory therapist measures the pH of her blood and determines that it is 7.15. Is the pH of her blood more acidic or more basic than normal? Has the number of hydrogen ions in her blood dcreased or decreased? Explainarrow_forward
- What effect is observed on the pH of the blood in each of the following cases? a. [HCO 3−] decreases.c. [CO 2] increases. b. [HCO 3−] increases.d. [CO 2] decreases.arrow_forwardWhat is the function of the buffer hemoglobin in the human body?arrow_forwardCarbon dioxide dissolved in body fluids has a pronounced effect on pH.(a) Does pH go up or down when carbon dioxide dissolves in these fluids? Does this change indicate higher or lower acidity?(b) What does a blood gas analysis measure?arrow_forward
- Considering the body’s pH balance is impacted by oxygen intake, why would the pH balance of blood plasma increase with rapid breathing? Conversely, why would the pH balance of blood plasma decrease if a person holds their breath?arrow_forwardwhat would happen topH if red blood cells could not synthesize bicarbonate?arrow_forwardPlasma contains more sodium than chloride. How can this be if individual ions of sodium and chloride exactly balance each other out, and plasma is electrically neutral?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning
Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning

Human Physiology: From Cells to Systems (MindTap ...
Biology
ISBN:9781285866932
Author:Lauralee Sherwood
Publisher:Cengage Learning

Biochemical Tests-Part 1; Author: Southern Stacker;https://www.youtube.com/watch?v=a-i9vANfQWQ;License: Standard Youtube License