
Principles of Instrumental Analysis
7th Edition
ISBN: 9781305577213
Author: Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 25, Problem 25.10QAP
Quinone undergoes a reversible reduction at a voltammetric working electrode. The reaction is
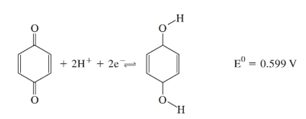
(a) Assume that the diffusion coefficients for quinone and hydroquinone are approximately the same and calculate the approximate half-wave Potential (versus SCE) for the reduction of hydroquinone at an RDE from a solution buffered to a pH of 8.0.
(b) Repeat the calculation in (a) for a solution buffered to a pH of 5.0.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Outline the dissociation reactions for carbonic acid and list the electrical charges of protonated and deprotonated forms.
Attach a plot for the portions of molecules possessing each of the outlined charges as function of pH.
The oxidation step uses a palladium salt. Suggest a mechanism for this coupling, which you have not encountered. Hint: Do not concern yourself with the role of the metal except as an acceptor of electrons
The literature indicate a difficult oxidation, but do suggest the formation of acetic and formic acid due to cleavage. The answer provided do not make any mention of this?
Please explain
Chapter 25 Solutions
Principles of Instrumental Analysis
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is your observation if some starch are added to the electrolyzed solution? Explain and discuss at which electrode this is happened?arrow_forwardWhat is the peroxide effect? What does it mean when there is a reversal of orientation? Detailed answers are appreciated. Thanks in advance!arrow_forwardComment on the above reaction with the largest conductivity change. Explain what is happening at the molecular level to have such a large increase in codutivityarrow_forward
- Write are the mechanism of reference electrode for maintaining constant potential? answer at your own words and answer as short as possible.arrow_forwardA molecule analogous to camphor is 4-t-butyl- cyclohexanone, seen below. Speculate on whether the exo or endo pathway would be predominate during a reduction reaction with hydride ion and explain your answer clearly.arrow_forwardPlease don't provide handwritten solution .... what is the written out electron pushing mechanism for thisarrow_forward
- K2Cr2O7 in acidic medium oxidises into which compound by H2O2 ?arrow_forwardDraw and propose simple schemes of oxidation and/or reduction mechanisms by which this compound may undergo (consider chemoselectivity). Explain briefly the mechanisms.arrow_forwardWhere does the strongest IMF occur in liquid ethanol?arrow_forward
- Investigate the solubility of maleic anhydride, maleic acid to fumaric acid and make comparisons.arrow_forwardCalculate the standard free energy change (AG") for the transfer of electrons from succinate to ubiquinone: Succinate + ubiquinone O -2.7 kJ/mol relevant half-reactions and standard reduction potentials: fumarate + 2H* + 2 e™ H succinate ubiquinone + 2 H+ + 2 e² = O2.7 kJ/mol -8.7 kJ/mol O 14.7 kJ/mol succinate dehydrogenase O -6.0 kJ/mol Fumarate + ubiquinol ubiquinol E = 0.031 V ED - 0.045 V Atte 1 Harrow_forwardChemistry provide missing products and outline electron pusbing mechanism for the reaction.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemical Principles in the LaboratoryChemistryISBN:9781305264434Author:Emil Slowinski, Wayne C. Wolsey, Robert RossiPublisher:Brooks Cole
Chemical Principles in the LaboratoryChemistryISBN:9781305264434Author:Emil Slowinski, Wayne C. Wolsey, Robert RossiPublisher:Brooks Cole Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Brooks Cole


Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning
O-Level Chemistry | 16 | Qualitative Analysis [1/3]; Author: Bernard Ng;https://www.youtube.com/watch?v=oaU8dReeBgA;License: Standard YouTube License, CC-BY