
Concept explainers
(a)
Interpretation:
The product formed by the reaction of D-galactose with
Concept Introduction:
The reaction of
(a)
Explanation of Solution
The D-galactose is an aldohexose molecule. The carbonyl group present in D-galactose is
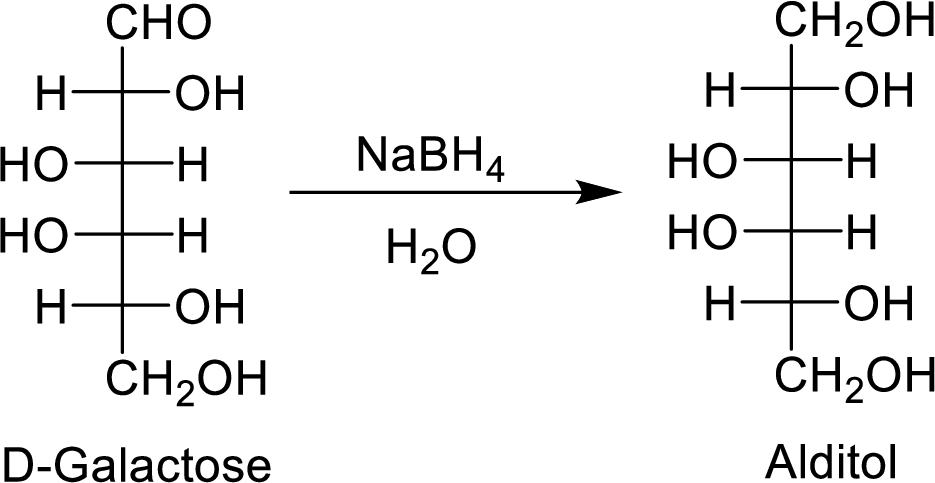
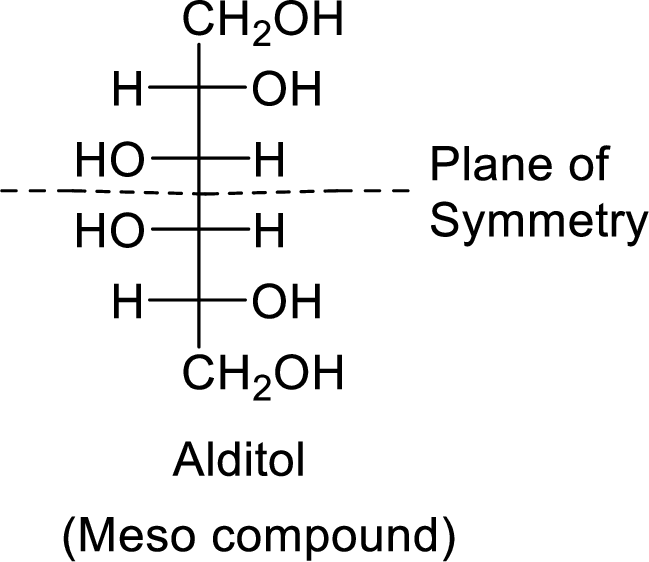
The formed alditol is optically inactive as it is a meso compound and shows plane of symmetry.
(b)
Interpretation:
The product formed by the reaction of D-galactose with
Concept Introduction:
The reagent
(b)
Explanation of Solution
When the D-galactose molecule is made to react with
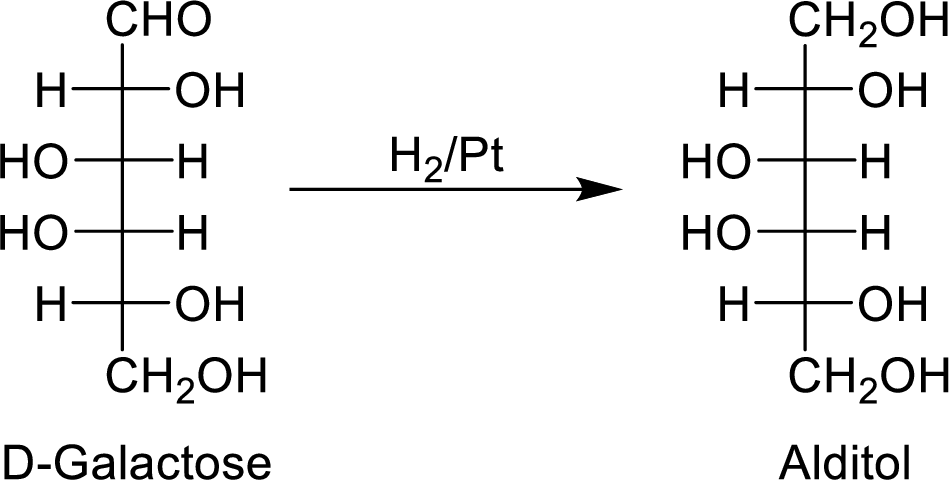
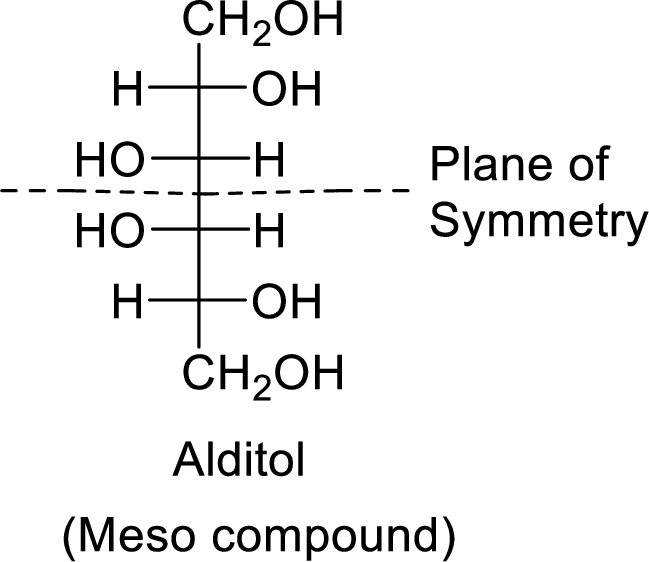
The formed alditol is optically inactive as it is a meso compound and it shows plane of symmetry.
(c)
Interpretation:
The product formed by the reaction of D-galactose with warm
Concept Introduction:
The reaction of warm
(c)
Explanation of Solution
The nitric acid (
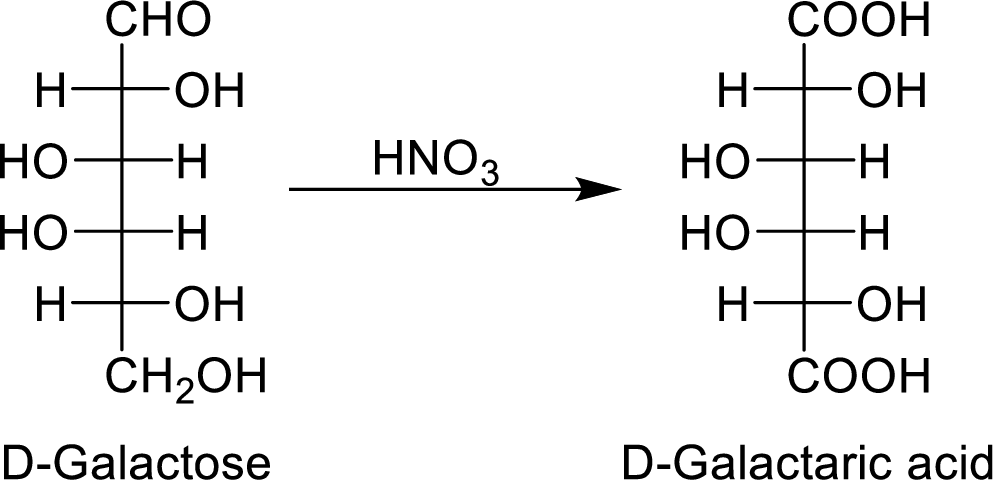
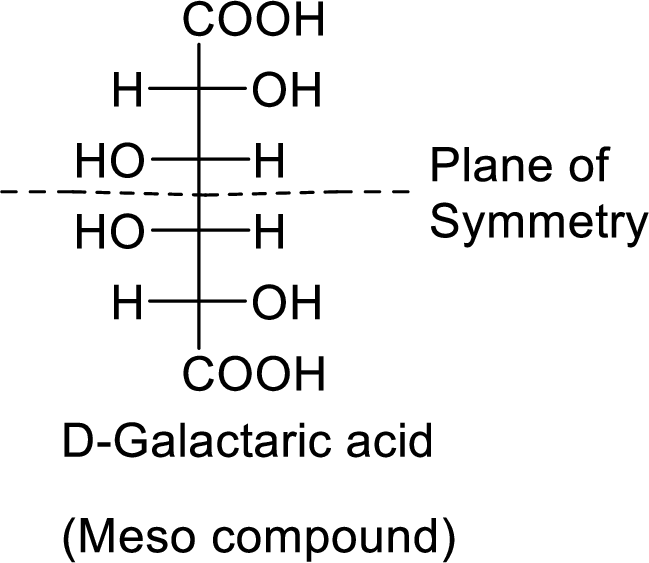
The product formed is a meso compound and shows plane of symmetry. So, it is optically inactive.
(d)
Interpretation:
The product formed by the reaction of D-galactose with
Concept Introduction:
The reaction of
(d)
Explanation of Solution
When the D-galactose reacts with
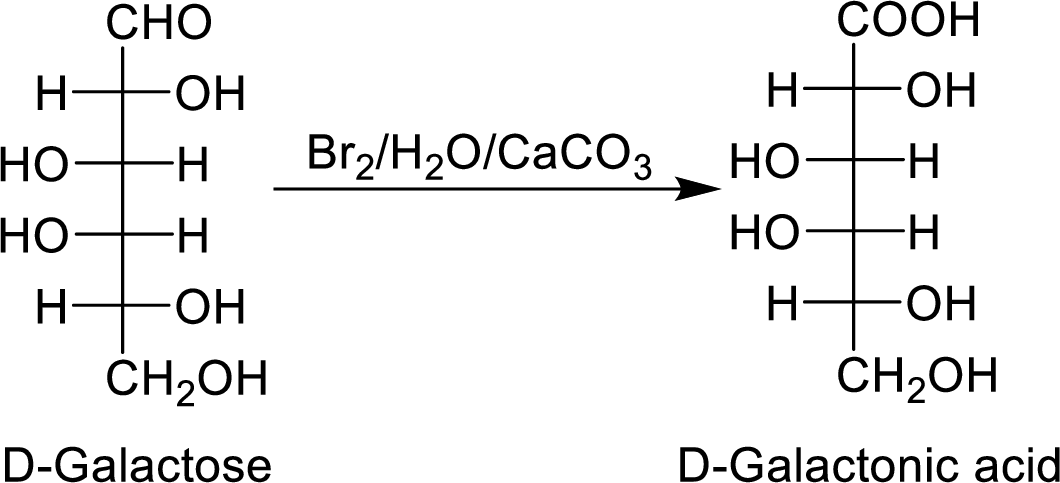
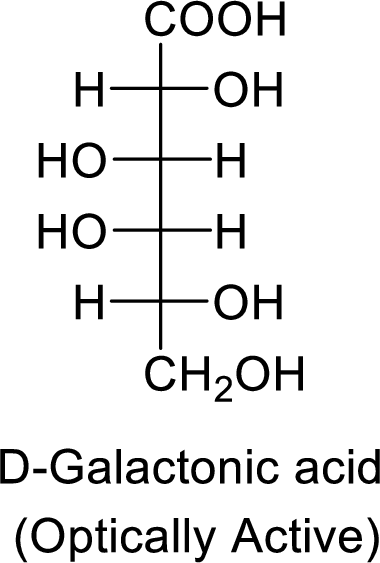
The formed product is optically active.
(e)
Interpretation:
The product formed by the reaction of D-galactose with
Concept Introduction:
The reaction of
(e)
Explanation of Solution
The D-galactose molecule consumes
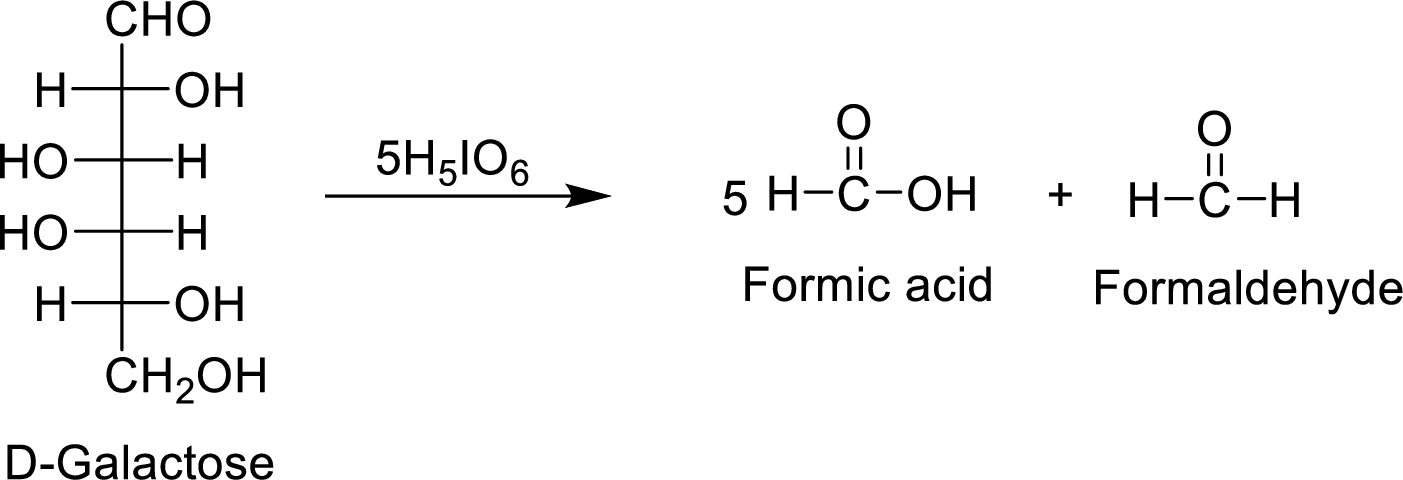
The products formed are achiral and are optically inactive.
(f)
Interpretation:
The product formed by the reaction of D-galactose with aniline (
(f)
Explanation of Solution
The D-galactose molecule reacts with aniline molecule and the aldehyde group of carbohydrate is reacted with the
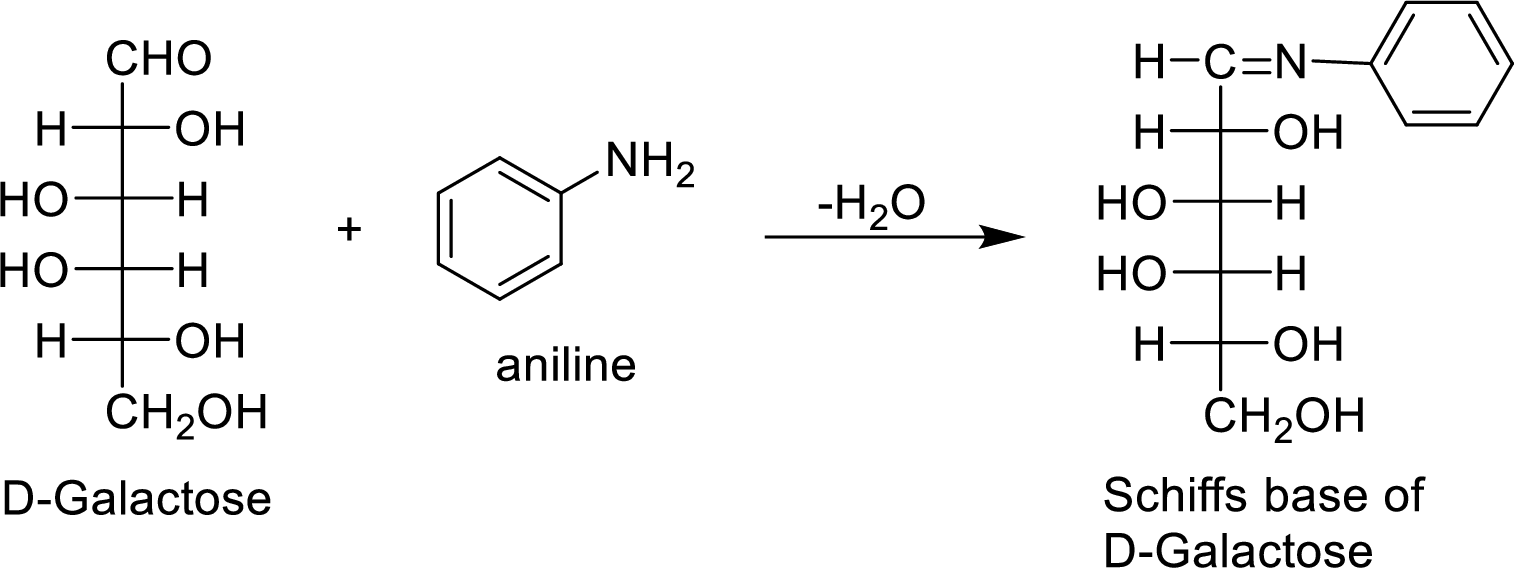
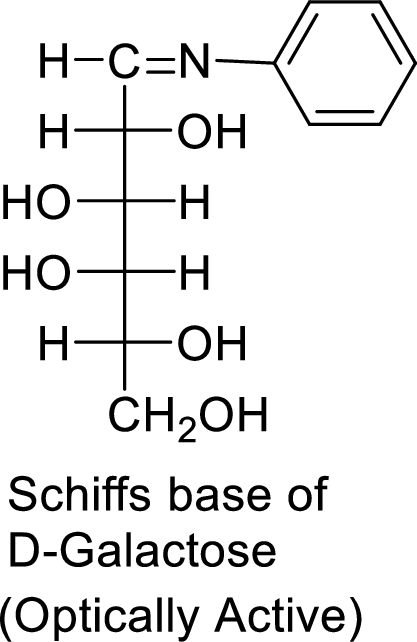
The formed product is a chiral compound and optically active.
Want to see more full solutions like this?
Chapter 25 Solutions
Organic Chemistry
- Draw Fischer projections for the product(s) formed by reaction of d-ribose with the following. In addition, state whether each product is optically active or inactive Q. HNO3, warmarrow_forwardRaffinose is a trisaccharide (C18H32O16) isolated from cottonseed meal. Raffinose doesnot reduce Tollens reagent, and it does not mutarotate. Complete hydrolysis of raffinosegives d-glucose, d-fructose, and d-galactose. When raffinose is treated with invertase,the products are d-fructose and a reducing disaccharide called melibiose. Raffinose isunaffected by treatment with a b@galactosidase, but an a@galactosidase hydrolyzes itto d-galactose and sucrose. When raffinose is treated with dimethyl sulfate and basefollowed by hydrolysis, the products are 2,3,4-tri-O-methylglucose, 1,3,4,6-tetraO-methylfructose, and 2,3,4,6-tetra-O-methylgalactose. Determine the completestructures of raffinose and melibiose, and give a systematic name for melibiose.arrow_forwardAn unknown reducing disaccharide is found to be unaffected by invertase enzymes. Treatment with an a@galactosidasecleaves the disaccharide to give one molecule of d-fructose and one molecule of d-galactose. When the disaccharideis treated with excess iodomethane and silver oxide and then hydrolyzed in dilute acid, the products are2,3,4,6-tetra-O-methylgalactose and 1,3,4-tri-O-methylfructose. Propose a structure for this disaccharide, and give itscomplete systematic name.arrow_forward
- Draw the Fischer projection of the product of the reduction reaction of D-Talose at C1C1. Drag the appropriate labels to their respective targets.arrow_forwardD-Tagatose is epimeric of D-Fructose at C4. What is the structure of α-D-Tagatofuranose?arrow_forwardIllustrate (hand-drawn) the mechanism of intrahemiacetal or intrahemiketal formation in the following monosaccharides and identify/draw the possible products of these reactions. A. D-talose B. D-fructosearrow_forward
- A D-aldohexose A is formed from an aldopentose B by the Kiliani–Fischer synthesis. Reduction of A with NaBH4 forms an optically inactive alditol. Oxidation of B forms an optically active aldaric acid. What are the structures of A and B?arrow_forwarda). The Fischer projection of D-Galactose is given below. Draw Haworthprojections for the a and b anomers (both cyclic hemiacetals) of D-Galactose.arrow_forwardAn oligosaccharide isolated from an organism is found tocontain two glucose residues and one galactose residue.Exhaustive methylation followed by hydrolysis producedtwo glucoses with methoxy groups at positions 2, 3,and 6 and galactose with methoxy groups at positions2, 3, 4, and 6. What is the structure of the originaloligosaccharide?arrow_forward
- Trehalose, C12H22O11, is a nonreducing sugar that is only 45% as sweet as sugar. When hydrolyzed by aqueous acid or the enzyme maltase, it forms only d-glucose. When it is treated with excess methyl iodide in the presence of Ag2O and then hydrolyzed with water under acidic conditions, only 2,3,4,6-tetra-O-methyl-d-glucose is formed. Draw the structure of trehalosearrow_forwardis Gulose is a c4 epimer of galactose? What should be their relationship?arrow_forwardThe carbonyl group in d-galactose may be isomerized from C1 to C2 by brief treatmentwith dilute base (by the enediol rearrangement, Section 23-7). The product is the C4epimer of fructose. Draw the furanose structure of the product.arrow_forward

 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT

