
Concept explainers
Interpretation:
Using octadecanoic (stearic) acid and any necessary organic and inorganic reagents, an efficient synthesis for each compound is to be described.
Concept introduction:
The reduction of carboxylic acid gives primary alcohol by using reducing agent lithium aluminum hydride
The primary alcohol on oxidation with pyridinium dichromate
In Clemmensen reduction, the carbonyl group (aldehyde or
The esters can be synthesized by acid catalyzed condensation of carboxylic acid with alcohol.
The ester on reaction with one molar equivalent of Grignard’s reagent in diethyl ether gives ketone by carbon-carbon bond formation.
The ester on reaction with two molar equivalents of Grignard’s reagent in diethyl ether gives tertiary alcohol.
The dehydration of alcohol is the loss of
The alcohol on acid catalyzed dehydration gives corresponding alkene.
The alkene on hydrogenation with the catalyst undergoes addition of hydrogen across the double bond and forms an alkane.
The primary amine can be prepared by the acylation of ammonia.
The secondary amide can be prepared by the nucleophilic substitution of acyl chloride by amine. The two moles amines used with one mole of acyl chloride, because one amine molecule acts as a nucleophile and second acts as a Brønsted base.
The carboxylic acids on reaction with thionyl chloride forms acyl chloride by replacing the hydroxyl group of carboxylic acid with chlorine atom.
The primary amide on reduction with lithium aluminum hydride
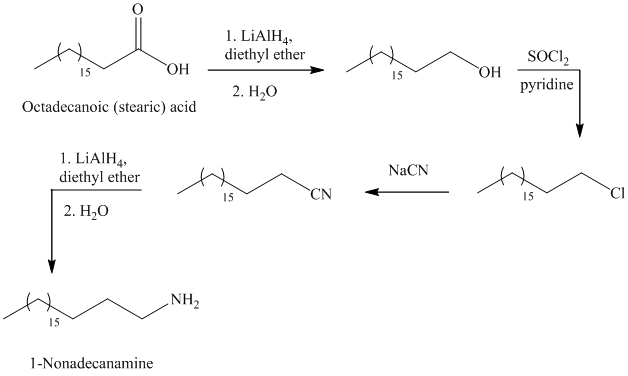
The reaction of thionyl chloride with alcohol gives alkyl halide.
The reaction of alkyl halide with sodium cyanide gives alkyl cyanide.
The cyanide (nitrile) can be reduced to primary amine using lithium aluminum hydride
The alkyl bromide can be prepared by the reaction of alcohol with phosphorus tribromide
Grignard reagents are prepared by the reaction of the magnesium metal with an alkyl or aryl halide usually in diethyl ether as the solvent.
The potassium or sodium dichromate in presence of strong acid forms chromic acid which is a good oxidizing agent, in hydrous medium oxidizes primary alcohol to carboxylic acid.
The epoxide on treatment with Grignard reagent undergoes epoxide ring opening by forming a corresponding alcohol.
Answer to Problem 28P
Solution:
a)
b)
c)
d) 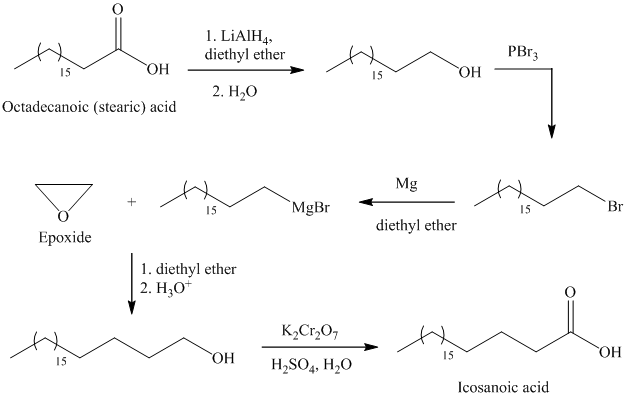
e) 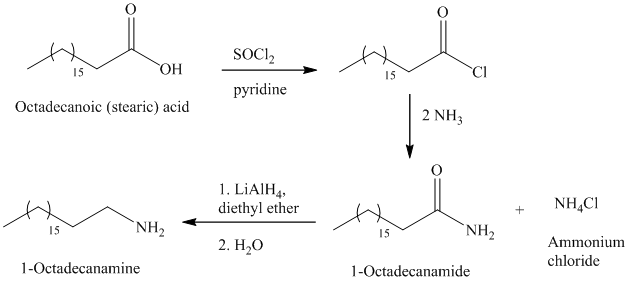
f)
Explanation of Solution
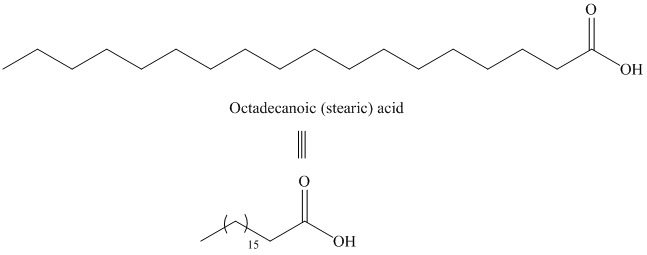
The structure of octadecanoic (stearic) acid is shown below:
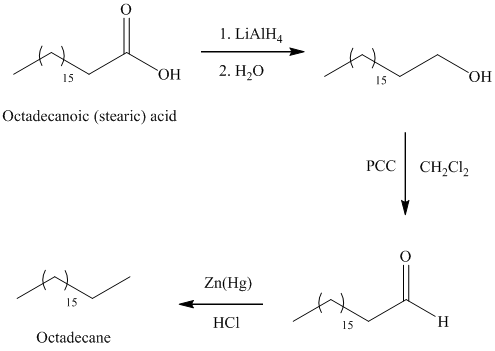
a) Octadecane
The synthesis of octadecane from octadecanoic acid can be done by following reactions sequence:
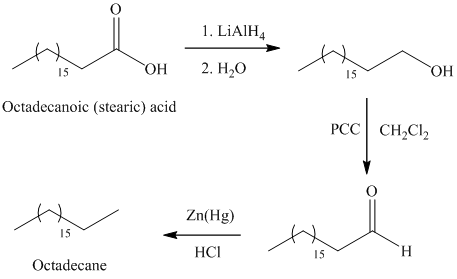
The octadecanoic acid on reaction with lithium aluminum hydride in aqueous medium reduced to octadecanol which is further on oxidation with pyridinium dichromate
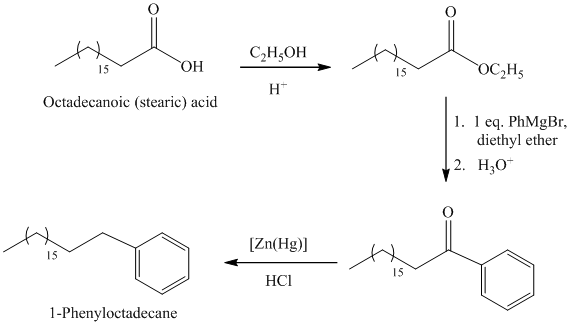
b)
The synthesis of
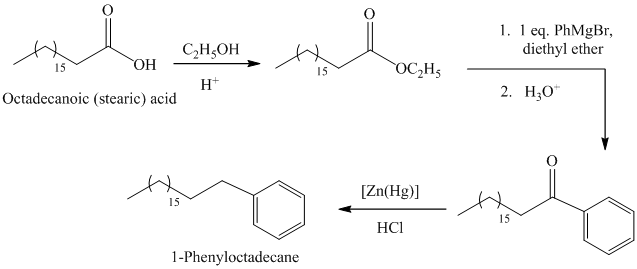
The octadecanoic acid first converted to an ester by reacting it with ethanol in acidic condition. The ester formed is then reacted with a Grignard’s reagent phenylmagnesium bromide
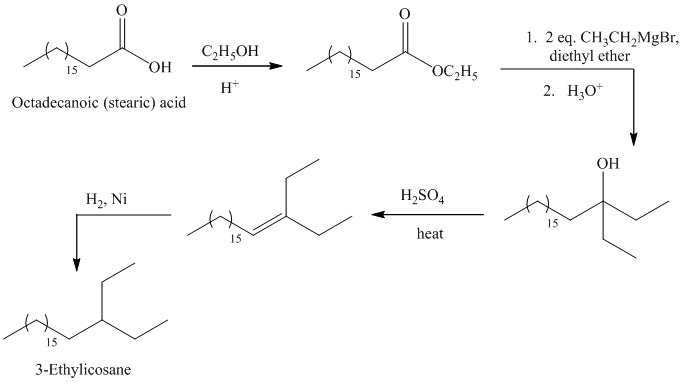
c)
The synthesis of
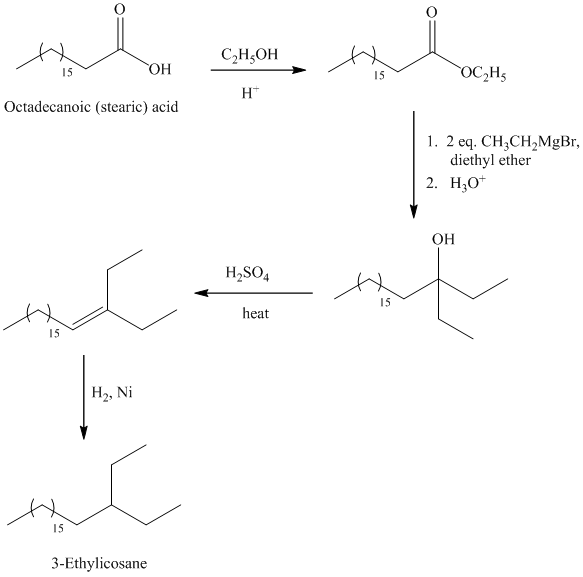
The octadecanoic acid is first converted to an ester by reacting it with ethanol in acidic condition. The ester formed is then reacted with a Grignard’s reagent ethyl bromide bromide
d) Icosanoic acid
The synthesis of Icosanoic acid from octadecanoic acid can be done by following reactions sequence:
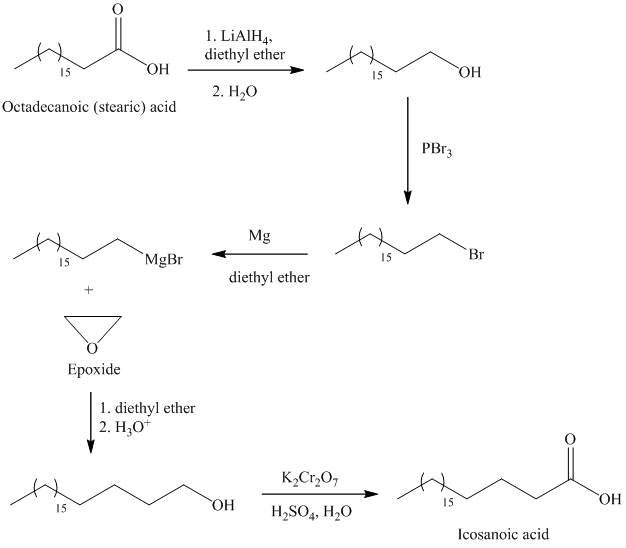
In the first step, the octadecanoic acid is reduced to primary alcohol by reducing agent lithium aluminum hydride
e)
The synthesis of
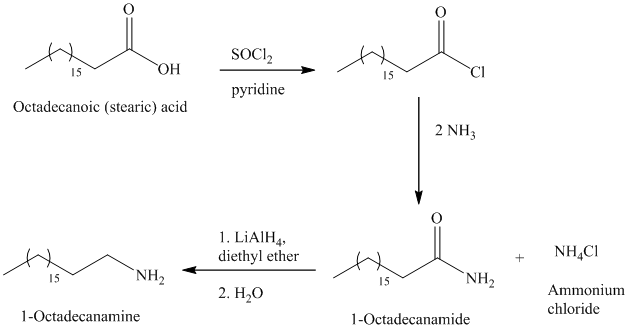
The octadecanoic acid on reaction with thonyl chloride in presence of pyridine gave the product of acyl chloride. The acyl chloride is converted to
f)
The synthesis of
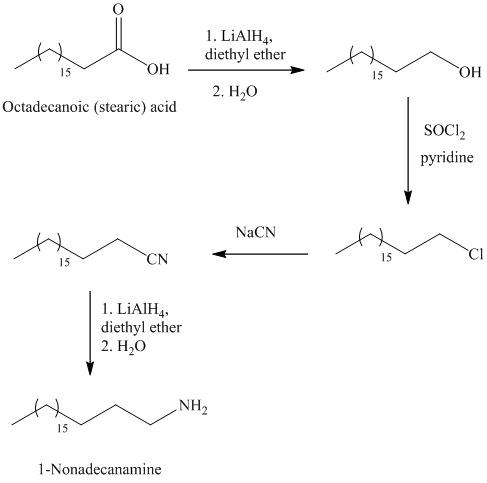
In the first step, the octadecanoic acid is reduced to primary alcohol by reducing agent lithium aluminum hydride
Want to see more full solutions like this?
Chapter 25 Solutions
ORGANIC CHEMISTRY-PACKAGE >CUSTOM<
- Write the structure of the principal organic product obtained on nitration of each of the following: (a) p-Methylbenzoic acid (d) p-Methoxyacetophenone (b) m-Dichlorobenzene (e) p-Methylanisole (c) m-Dinitrobenzene (f ) 2,6-Dibromoanisolearrow_forwardWhat aldehyde or ketone and nitrogen component are needed to synthesize N-ethylcyclohexanamine by a reductive amination reaction?arrow_forward1. ) 2-Pentanol when introduced with H2SO4 will become ____________ . H2SO4 is a Dehydrating agent (used the concept of Zaitsev to come up with the major product)1-Pentene 1-Pentanal2-Pentene2- Pentanone2.) Diabetic breath has a mild sweet odor because of AroamticAldehydeAlkeneKetone3.) The following compounds contain a Carbonyl group, exceptAcetaminophenCitric AcidPhenolDacroonarrow_forward
- How would you recover Benzoic acid from a mixture containing P-Chloroaniline and Biphenyl . Provide a balanced chemical equation for the reaction . Indicate physical states. Provide structures but not the molecular formula.arrow_forwardWrite the equations to show how isobutyric acid could be converted to each of the following using needed reagentsa. magnesium isobutyrateb. isobutyryl chloridec. isobutyl alcohold. ethyl isobutyratee. isobutyramidearrow_forwardPredict the chemical name of compound B a. p-chlorobenzylamine b. 4-bromoaniline c. benzylamine d. p-chloronitrile e. p-chlorobenzaldehyde 2. What is the reaction name for the chemical transformation of A to B a. reductive amination b. catalytic reduction c. carbonyl dehydration d. Hofmann elimination e. Aldehyde rearrangementarrow_forward
- Preamble: A student chemist in an attempt to synthesise compound B from the aromatic aldehyde. 24. What is the reaction name for the chemical transformation of A to BA. reductive aminationB. catalytic reductionC. carbonyl dehydrationD. Hofmann eliminationE. Aldehyde rearrangementarrow_forwardFollowing are structural formulas for two more widely used sulfonylurea hypoglycemic agents.Show how each might be synthesized by converting an appropriate amine to a carbamic ester and then treating the carbamate with the sodium salt of a substituted benzenesulfonamide.arrow_forwardAcetic anhydride is hydrolyzed by water to provide two molecules of acetic acid. Propose amechanism for this reaction.arrow_forward
- Synthesising menthone is an exothermic process that includes a reflux procedure. Mark the correct statements. * A- The reflux process enables us to transfers energy to the reaction mixture for an extended period of time without loss of solvent or reagents. B- The reflux process increases the rate of the oxidation of menthol. C- The reflux process increases the yield of the oxidation of menthol. D- A water bath can be used in the preparation of menthone because the boiling point of the reaction mixture is below 100°C due to the inclusion of acetone in this mixture.arrow_forward12. You can distinguish epinephrine hydrotartrate from norepinephrine hydrotartrate by? A. Water solubility B. Reactions of oxidation with iodine at different pH C. Reactions in general alkaloid precipitation reagents D. Reactions with iron(III) chloride E. Reactions in Fehling's reagent.arrow_forwardplease help with this question. thank you. n-acetylazoles go through hydrolysis more than regular amides. propose a reason for the reactivity of n-acetylazoles toward nucleophilic substitution.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

