
Concept explainers
(a)
Interpretation:
The role of the process of diffusion in the transport of gases through the body needs to be explained.
Concept Introduction:
The gasses are transported from the lungs to the whole body and this transfer of gases in the lungs takes place by the process of diffusion. The gases in the lungs diffuse from their region of high pressure to their region of low pressure and are either taken throughout the body or exhale out depending upon the gas.
(b)
Interpretation:
The role of carbaminohemoglobin in the transport of gases throughout the body needs to be explained.
Concept Introduction:
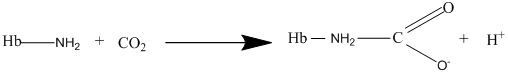
Other than diffusion, there are other methods for the transport of gases. The carbaminohemoglobin is one of the methods of transport for the carbon dioxide gas where the carbon dioxide binds with hemoglobin to form carbaminohemoglobin as per the following equation:
(c)
Interpretation:
The role of alveoli in the transfer of gases throughout the body needs to be explained.
Concept Introduction:
Alveoli are the air sacs in the lungs which basically increases the surface area capacity of the lungs and these alveoli are the sites of the gases transfer from the atmosphere to the blood in the body.
(d)
Interpretation:
The role of carbonic anhydrase in the transfer of gases throughout the body needs to be explained.
Concept Introduction:
The transfer of carbon dioxide also takes place by means of carbonic anhydrase. The carbonic anhydrase converts to carbonic acid

Want to see the full answer?
Check out a sample textbook solution
Chapter 25 Solutions
General, Organic, & Biological Chemistry
- Consider this question: What is the molecular formula of a compound that contains 39% C. 45% N, and 16% H if 0.157 g of the compound occupies 125 mL with a pressure of 993 kPa at 22 C? (a) Outline the steps necessary to answer the question. (b) Answer the question.arrow_forward5.32 Cylinders of compressed gases are often labeled to show how many “SCF” or “standard cubic feet” of gas they contain. 1 SCF of gas occupies a volume of 1 ft3 at a standard temperature and pressure of 0°C and 1 atm. A particular cylinder weighs 122 lb. when empty and 155 lb. when filled with krypton gas at 26°C. How many SCF of Kr does this cylinder contain?arrow_forwardWhen hydrogen peroxide decomposes, oxygen is produced: 2H2O2(aq)2H2O+O2(g)What volume of oxygen gas at 25C and 1.00 atm is produced from the decomposition of 25.00 mL of a 30.0% (by mass) solution of hydrogen peroxide (d=1.05g/mL)?arrow_forward
- 51 What volume of oxygen at 24 C and 0.88 atm is needed to completely react via combustion with 45 g of methane gas?arrow_forward5.34 Define the term mole fractionarrow_forwardNitrogen gas can be obtained by decomposing ammonium nitrate at high temperatures. The nitrogen gas is collected over water in a 500-mL (three significant figures) flask at 19C. The ambient pressure is 745 mm Hg. (Vapor pressure of water at 19C is 16.48 mm Hg.) (a) What is the partial pressure of nitrogen? (b) How many moles of water are there in the wet gas? (c) How many moles of dry gas are collected? (d) If 0.128 g of Ne are added to the flask at the same temperature, what is the partial pressure of neon in the flask? (e) What is the total pressure after Ne is added?arrow_forward
- Helium gas, He, at 22C and 1.00 atm occupied a vessel whose volume was 2.54 L. What volume would this gas occupy if it were cooled to liquid-nitrogen temperature (197C)?arrow_forwardA mixture of 0.200 g of 1.00 g of and 0.820 g of Ar is stored in a closed container at STP. Find the volume of the container, assuming that the gases exhibit ideal behavior.arrow_forwardHow many liters of gas can be collected over water 55 degrees and 1.6 atm when excess phosphoric acid is reacted with 25 grams of cupric sulfite?arrow_forward
- Infer why gases such as the oxygen used at hospitals are compressed. Why mustcompressed gases be shielded from high temperatures? What must happen tocompressed oxygen before it can be inhaled?arrow_forwardThe active ingredient of Benadryl© Chesty Forte Cough Liquid is Guaiphenesin anexpectorant drug used to assist the expectoration ('bringing up') of phlegm from theairways in acute respiratory tract infections. A 200.0 mL bottle of Benadryl© ChestyForte Cough contains 4.00 g of Guaiphenesin (C10H14O4). i. If the recommended dose for a child is 150 mg of Guaiphenesin. What volume of thecough liquid should be administered? ii. If the recommended dose for an adult is 350 mg of Guaiphenesin. How many molesof Guaiphenesin does this equal?arrow_forwardThe active ingredient of Benadryl© Chesty Forte Cough Liquid is Guaiphenesin anexpectorant drug used to assist the expectoration ('bringing up') of phlegm from theairways in acute respiratory tract infections. A 200.0 mL bottle of Benadryl© ChestyForte Cough contains 4.00 g of Guaiphenesin (C10H14O4). i. If the recommended dose for a child is 150 mg of Guaiphenesin. What volume of thecough liquid should be administered? ii. If the recommended dose for an adult is 350 mg of Guaiphenesin. How many molesof Guaiphenesin does this equal? (answer to 2 significant figures)arrow_forward
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStaxChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStaxChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





