
Concept explainers
(a)
Interpretation: To determine whether glutamate and aspartate could function as the two reactants in a transamination reaction or not.
Concept introduction: Transamination reaction is a biochemical reaction that involves the transfer of an amino group. In transamination reaction exchange of an amino group from an
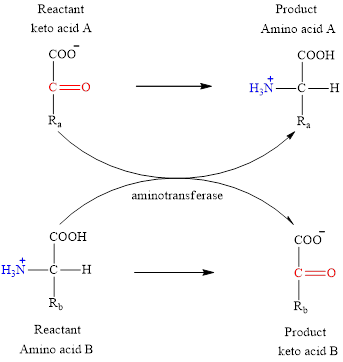
The general structure of an amino acid is:
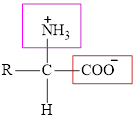
Here,
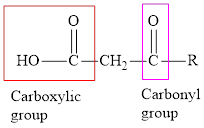
An acid containing both carbonyl and carboxyl functional group is known as a keto acid. A general representation of a keto acid is:
(a)
Answer to Problem 26.34EP
No, glutamate and aspartate cannot function as the reactants in a transamination reaction.
Explanation of Solution
Glutamate is an amino acid and its structure is:
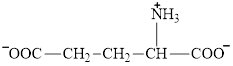
Aspartate is an amino acid and its structure is:
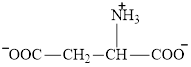
The two reactants in transamination reaction are a keto acid and an amino acid. Both glutamate and aspartate are amino acids thus they cannot function as reactants in a transamination reaction. For a transamination reaction to take place there must be one amino acid present along with a keto acid.
(b)
Interpretation: To determine whether aspartate and
Concept introduction: Transamination reaction is a biochemical reaction that involves the transfer of an amino group. In transamination reaction exchange of an amino group from an

The general structure of an amino acid is:

Here,
An acid containing both carbonyl and carboxyl functional group is known as a keto acid. A general representation of a keto acid is:

(b)
Answer to Problem 26.34EP
Yes, aspartate and
Explanation of Solution
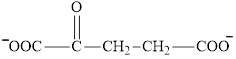
Aspartate is an amino acid and its structure is:
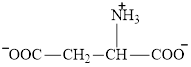
Transamination reaction involves the exchange of an amino group from an
(c)
Interpretation: To determine whether succinate and
Concept introduction: Transamination reaction is a biochemical reaction that involves the transfer of an amino group. In transamination reaction exchange of an amino group from an

The general structure of an amino acid is:

Here,
An acid containing both carbonyl and carboxyl functional group is known as a keto acid. A general representation of a keto acid is:

(c)
Answer to Problem 26.34EP
No, succinate and
Explanation of Solution
Succinate is a diacid acid and its structure is:
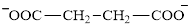
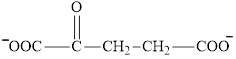
The two reactants in transamination reaction are a keto acid and an amino acid.
(d)
Interpretation: To determine whether glutarate and aspartate could function as the two reactants in a transamination reaction or not.
Concept introduction: Transamination reaction is a biochemical reaction that involves the transfer of an amino group. In transamination reaction exchange of an amino group from an
The general reaction to illustrate transamination is as follows:

The general structure of an amino acid is:

Here,
An acid containing both carbonyl and carboxyl functional group is known as a keto acid. A general representation of a keto acid is:

(d)
Answer to Problem 26.34EP
No, glutarate and aspartate cannot function as the reactants in a transamination reaction.
Explanation of Solution
Aspartate is an amino acid and its structure is:
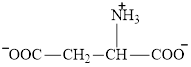
Glutarate is a diacid and its structure is:
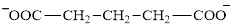
The two reactants in transamination reaction are a keto acid and an amino acid. Aspartate is an amino acid but glutarate is not a keto acid. For a transamination reaction to take place there must be one keto acid present along with an amino acid. Thus, glutarate and aspartate cannot function as the reactants in a transamination reaction.
Want to see more full solutions like this?
Chapter 26 Solutions
General, Organic, and Biological Chemistry
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co




