
(a)
Interpretation: The product formed from the ring-closing metathesis of given compound is to be drawn, and the synthesis of metathesis starting material using
Concept introduction: The ring-closing metathesis (RCM) by Grubbs catalyst occurs, when the starting material is diene. This reaction is facilitated under high-dilution condition as it favors intramolecular metathesis instead of intermolecular metathesis.
Answer to Problem 26.48P
The product formed from the ring-closing metathesis of given compound is,
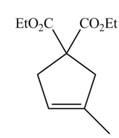
The synthesis of metathesis starting material using
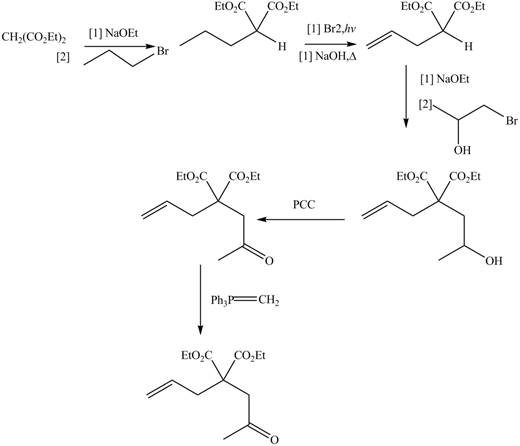
Explanation of Solution
The given compound is a diene.
The ring-closing metathesis (RCM) by Grubbs catalyst occurs, when the starting material is diene. This reaction is facilitated under high-dilution condition as it favors intramolecular metathesis instead of intermolecular metathesis.
The product formed from the ring-closing metathesis of given compound is drawn in Figure 1.
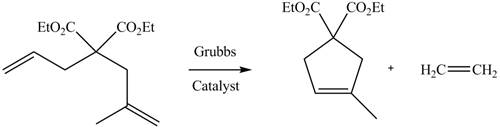
Figure 1
The synthesis of metathesis starting material involves five steps. The first step is reaction of
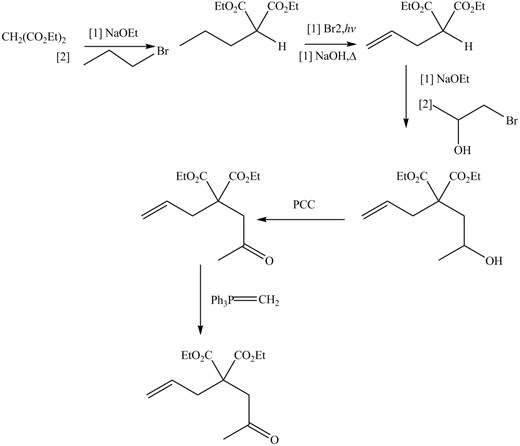
Figure 2
The product formed from the ring-closing metathesis of given compound is drawn in Figure 1. The synthesis of metathesis starting material using
(b)
Interpretation: The product formed from the ring-closing metathesis of given compound is to be drawn, and the synthesis of metathesis starting material using
Concept introduction: The ring-closing metathesis (RCM) by Grubbs catalyst occurs, when the starting material is diene. This reaction is facilitated under high-dilution condition as it favors intramolecular metathesis instead of intermolecular metathesis.
Answer to Problem 26.48P
The product formed from the ring-closing metathesis of given compound is,
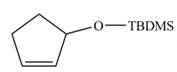
.The synthesis of metathesis starting material using
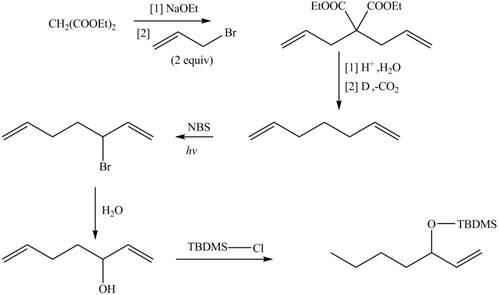
Explanation of Solution
The given compound is a diene.
The ring-closing metathesis (RCM) by Grubbs catalyst occurs, when the starting material is diene. This reaction is facilitated under high-dilution condition as it favors intramolecular metathesis instead of intermolecular metathesis.
The product formed from the ring-closing metathesis of given compound is drawn in Figure 3.
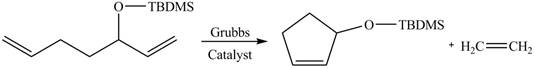
Figure 3
The synthesis of metathesis starting material involves five steps.The first step is reaction of
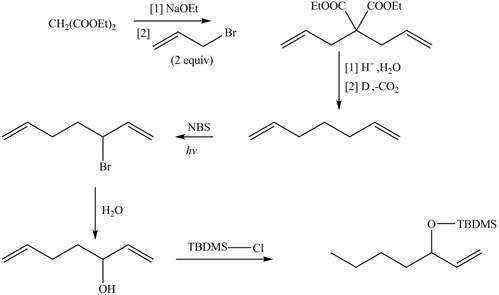
Figure 4
The product formed from the ring-closing metathesis of given compound is drawn in Figure 3. The synthesis of metathesis starting material using
Want to see more full solutions like this?
Chapter 26 Solutions
ORG.CHEMISTRY W/ACCESS+MODEL KIT PKG
- Devise a synthesis of each compound using CH3CH2CH=CH2 as the starting material. You may use any other organic compounds or inorganic reagents.arrow_forwardDraw the product formed from the ring-closing metathesis of each compound. Then, devise a synthesis of each metathesis starting material from benzene, alcohols with less than ve carbons, and any needed organic and inorganic reagents.arrow_forward(a) Give an acceptable name for each compound, (b) Draw the organic products formed when A or B is treated with each reagent: [1] H3O+; [2] −OH, H2O; [3] CH3CH2CH2MgBr (excess), then H2O; [4] LiAlH4, then H2O.arrow_forward
- Draw the product formed from the ring-closing metathesis of each compound. Then, devise a synthesis of each metathesis starting material using any of the following compounds: CH2(CO2Et)2, alcohols with less than ve carbons, and any needed organic and inorganic reagents.arrow_forwardDevise a synthesis of each alkene using a Wittig reaction to form the double bond. You may use benzene and organic alcohols having four or fewer carbons as starting materials and any required reagents.arrow_forwardDraw the product formed from the ring-closing metathesis of each compound. Then, devise a synthesis of each metathesis starting material from benzene, alcohols with four or fewer carbons, and any needed organic or inorganic reagents.arrow_forward
- Fill in the missing reagents a-h in the following scheme:arrow_forwardDevise a synthesis of each compound from CH3CH2CH2CO2Et, benzene, andalcohols having ≤ 2 C's. You may also use any required organic or inorganic reagents.arrow_forwardDevise a synthesis of dodec-7-yn-5-ol from hex-1-ene(CH3CH2CH2CH2CH=CH2) as the only organic starting material. You mayuse any other needed reagents.arrow_forward
- Devise a synthesis of each compound using 1-bromobutane(CH3CH2CH2CH2Br) as the only organic starting material. You may useany other inorganic reagents.arrow_forwardIn addition to using CHX3 and base to synthesize dihalocarbenes (Section 26.4), dichlorocarbene (:CCl2) can be prepared by heating sodium trichloroacetate. Draw a stepwise mechanism for this reaction.arrow_forwardDraw the product formed when (CH3)2CHOH is treated with each reagent. a. SOCl2, pyridine b. TsCl, pyridine c. H2SO4 d. HBr e. PBr3, then NaCN f. POCl3, pyridinearrow_forward
