
Concept explainers
The phosphorylation of
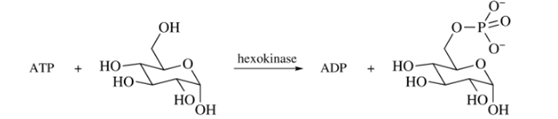
Is this reaction exergonic or endergonic?
How would the value of
Use the value for the hydrolysis of ATP to ADP (Section 27.5) to calculate
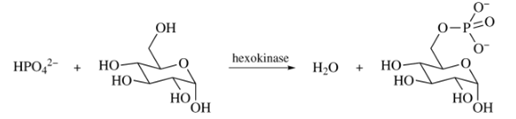
Want to see the full answer?
Check out a sample textbook solution
Chapter 27 Solutions
ORGANIC CHEMISTRY (LL)-W/SOLN.>CUSTOM<
- 1. How important is the value of free energy (∆G) in the spontaneity of chemical reactions? 2. How essential is energy coupling in the transfer of energy in the cells ?arrow_forwardAerobic respiration links the oxidation of glucose with the production of ATP form ADP.Given the following thermodynamic data, C6H12O6(s) + 6O2(g) → 6CO2(g) + 6H2O(l) ΔrH° = −2803 kJ/mol-rxn ATP + H2O(l) → ADP + HPO42-(aq) ΔrH° ≈ −24 kJ/mol-rxn calculate the enthalpy of reaction for the aerobic production of 25 ATP shown below.C6H12O6(s) + 6O2(g) + 25ADP + 25HPO42-(aq)→ 6CO2(g) + 25ATP + 31H2O(l) ΔrH° = ?arrow_forwardThe G° for the conversion of glucose 6-phosphate (G6P) to fructose 6-phosphate (F6P) is +1.7 kJ/mole. In a particular human cell the concentration G6P is 8.0 µM and the concentration F6P is 1.0 µM. Calculate the G of the reaction as it occurs in this cell at 37°C?arrow_forward
- Free energy changes under intracellular conditions differ markedly from those determined under standard conditions. ΔG°′ = -32.2 kJ>mol for ATP hydrolysis to ADP and Pi . Calculate ΔG for ATP hydrolysis in a cell at 37 °C that contains [ATP] = 3 mM, [ADP] = 1 mM, and [Pi] = 1 mM.arrow_forwardA 22g field mouse consumed 5g of mouse chow that had an energy content of 2Cal/g in 24hours andduring that time produced urine and feces with an energy content of 6Cal.a. What was the amount of energy that the mouse used that day?b. What is the field mouse’ mass specific metabolic rate in J/g*hr?c. If the mouse gained a gram of body mass over that same day would the metabolic ratecalculated be an underestimation or overestimation? Why?arrow_forwardMeasuring food consumption and excretion A 22g field mouse consumed 5g of mouse chow that had an energy content of 2Cal/g in 24hours andduring that time produced urine and feces with an energy content of 6Cal.a. What was the amount of energy that the mouse used that day?b. What is the field mouse’ mass specific metabolic rate in J/g*hr?arrow_forward
- In anaerobic bacteria, the source of carbon may be a molecule other than glucose and the final electron acceptor is some molecule other than O2. Could a bacterium evolve to use the ethanol/nitrate pair instead of the glucose/O2 pair as a source of metabolic energy?arrow_forwardIn glycolysis, the reaction of glucose (Glu) to form glucose-6-phosphate (G6P) requires ATP to be present as described by the following equation:Glu + ATP⟶G6P + ADP ΔG°298 = −17 kJIn this process, ATP becomes ADP summarized by the following equation:ATP⟶ADP ΔG°298 = −30 kJDetermine the standard free energy change for the following reaction, and explain why ATP is necessary to drive this process:Glu⟶G6P ΔG°298 = ?arrow_forwardCalculate the standard free energy change (ΔG°') for the reaction catalyzed by the enzyme aspartate amino transferase (K'eq = 6.8) using the given equilibrium constant for the reactions at 25°C and pH 7.0.arrow_forward
- Chemistry Calculate the standard free-energy for creatine-phosphate hydrolysis in a cell where: creatine-P = 60mmol, creatine = 30 mmol, ATP = 1 mmol, ADP = 0.1 mmol. Is this reaction exer/endergonic?arrow_forwardAt 25 oC an antibody binds a protein with a ∆H = -87.9 kJ/mol and ∆S = -118.9 J mol-1 K-1 Which of the following is/are true? (Choose all that are correct) a. The process is exergonic b. The process is endothermic c. The entropy of the binding process increases d. The binding process is exothermic e. The entropy during the binding process decreasesarrow_forwardThe ΔG°’ for binding a substrate to an enzyme is -14 kJ/mol at 25°C. What is the Keq’ for the reaction? R is 8.31 J/mol-K. in 3 sig.figs.arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





