
Concept explainers
Interpretation:
The given compounds caffeine and theobromine are to be classified as pyrimidine and purine. The fact that one of these two cannot isomerize to an enolic form, while two different enols are possible for the other is to be explained. The structural formulas for the possible enols is to be written.
Concept introduction:
The purines and pyrimidines are nitrogen bases that constitute the structural unit of
Both purines and pyrimidines are nitrogen containing heterocyclic
The structures of pyrimidine and purine are shown below:
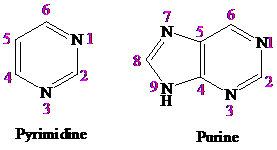
The structure of pyrimidine resembles that of benzene and pyridine having two nitrogen atoms.
In the structure of purine, the prymidine ring is fused with imidazole ring and has four nitrogen atoms.
The structure having an acidic hydrogen at alpha position to
Want to see the full answer?
Check out a sample textbook solution
Chapter 27 Solutions
Organic Chemistry (Binghampton University)
- Both norepinephrine and epinephrine are synthesized from the same protein-derived amino acid. From which amino acid are they synthesized? What types of reactions are involved in their biosynthesis?arrow_forwardCholic acid is secreted in bile as an amide linked to the aminogroup of glycine. This cholic acid–amino acid combination acts asan emulsifying agent to disperse lipids in the intestines for easierdigestion. Draw the structure of the cholic acid–glycine combination,and explain why it is a good emulsifying agent.arrow_forwardDraw the structure of the choline group in phosphatidylcholine (or give its stoichiometry)arrow_forward
- Write the therapeutic action of following on human body and mention the class of drugs to which each of these belong:(i) Ranitidine(ii) Morphine(iii) Aspirinarrow_forwardDraw out an appropriate chemical structure for the name provided. p-isopropylanilinearrow_forwardThe structure of a triacylglycerol molecule can be described in terms of the fatty acyl groups that appear at each of the three positions along the glycerol backbone. Human milk contains a triacylglycerol with an oleoyl residue at position 1, a palmitoyl residue at position 2, and a linoleoyl residue at position 3. Draw the structure of this molecule, using the information in the table below. For example, an oleoyl residue is derived from oleic acid.arrow_forward
- A tripeptide undergoes complete hydrolysis and the resulting mixture contains only phenylalanine and glycine. Draw all possible sequences for the original tripeptide.arrow_forwardWhat is the principle behind the isolation of albumin?arrow_forwardWhat are some common examples of homopolysaccharide and their importance?arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning



