
CHEM 262 ORG CHEM EBOOK DIGITAL DELIVERY
8th Edition
ISBN: 2818440043505
Author: Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 27.11, Problem 20P
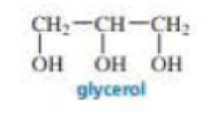
Explain why, when a small amount of glycerol is added to the reaction mixture of toluene-2,6-diisocyanate and ethylene glycol during the synthesis of polyurethane foam, a much stiffer foam is obtained.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
(b)
A researcher wishes to obtain a glycoside (an organic compound) from an aqueous plant extract
using the solvent extraction technique. She therefore performs the following steps:
Step 1: An aqueous solution (100 mL) containing 7 g of the glycoside was shaken with 50 mL of
ethyl acetate.
Step 2: The resulting aqueous layer from Step 1 was further shaken with 50 mL of
dichloromethane.
Given that:
Kp for the glycoside in ethyl acetate-water = 0.8
Kp for the glycoside in dichloromethane-water = 0.9
(i) Calculate the total mass of glycoside extracted.
(ii) If the molar mass of the glycoside = 784.3 g/mol, calculate the molar concentration of the
glycoside remaining in the aqueous layer after each extraction.
Restate the paragraph.
The main objective of the laboratory activity was to make aspirin also known as acetylsalicylic acid in its scientific name. It was successfully done as aspirin was synthesized using the combination of salicylic acid and acetic anhydride. In this reaction, salicylic acid's hydroxyl group on the benzene ring interacted with acetic anhydride to generate an ester functional group. This is an esterification reaction that produces acetylsalicylic acid. Sulfuric acid was utilized as a catalyst to start the esterification reaction. Some unreacted acetic anhydride and salicylic acid, as well as sulfuric acid, aspirin, and acetic acid, remained in the solution after the reaction was completed. The process of crystallization was used to obtain pure crystals of a substance from an impure mixture. Upon completion of the lab, analysis, and calculations, , it is evident that the synthesis of aspirin is possible using these methods but that the yield will be relatively low
write the mechanism with which toluene reacts with acetylchloride in the presence of aluminum trichloride. What is the name of this reaction?
Chapter 27 Solutions
CHEM 262 ORG CHEM EBOOK DIGITAL DELIVERY
Ch. 27.3 - Prob. 1PCh. 27.3 - Prob. 2PCh. 27.3 - Prob. 3PCh. 27.3 - Prob. 4PCh. 27.3 - Prob. 5PCh. 27.3 - Prob. 6PCh. 27.4 - Prob. 7PCh. 27.5 - Rank the following groups of monomers from most...Ch. 27.5 - Why does methyl methacrylate not undergo cationic...Ch. 27.6 - Prob. 10P
Ch. 27.6 - Explain why, when propylene oxide undergoes...Ch. 27.6 - Which monomer and which type of initiator can you...Ch. 27.6 - Prob. 13PCh. 27.8 - Draw a short segment of gutta-percha.Ch. 27.8 - Prob. 15PCh. 27.11 - Prob. 16PCh. 27.11 - Write an equation that explains what happens if a...Ch. 27.11 - What happens to polyester slacks if aqueous NaOH...Ch. 27.11 - a. Propose a mechanism for the formation of the...Ch. 27.11 - Explain why, when a small amount of glycerol is...Ch. 27.12 - Propose a mechanism for the formation of melmac.Ch. 27.12 - Prob. 22PCh. 27.13 - Prob. 23PCh. 27 - Draw short segments of the polymers obtained from...Ch. 27 - Prob. 25PCh. 27 - Prob. 26PCh. 27 - Draw the structure of the monomer or monomers used...Ch. 27 - Prob. 28PCh. 27 - Draw short segments of the polymers obtained from...Ch. 27 - Quiana is a synthetic fabric that feels very much...Ch. 27 - Prob. 31PCh. 27 - Prob. 32PCh. 27 - Prob. 33PCh. 27 - Poly(vinyl alcohol) is a polymer used to make...Ch. 27 - Five different repeating units are found in the...Ch. 27 - Prob. 37PCh. 27 - A particularly strong and rigid polyester used for...Ch. 27 - Prob. 39PCh. 27 - Which Monomer gives a greater yield of polymer,...Ch. 27 - Prob. 41PCh. 27 - Prob. 42PCh. 27 - Why do vinyl raincoats become brittle as they get...Ch. 27 - The polymer shown below is synthesized by...Ch. 27 - Prob. 45PCh. 27 - How can head-to-head poly(vinyl bromide) be...Ch. 27 - Delrin (polyoxymethylene) is a tough...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- in the synthesis of benzocaine: what is the structure of the precipitate that formed after the sulfuric acid was added to the cooled mixture of p-aminobenzoic acid and pure ethanol?arrow_forwardWrite the products that are formed in the reaction of tert-butyldiphenylchlorosilane with 1,3-pentanediol in a basic medium.arrow_forwardDescribes the procedure for discarding Isoflurane after using it.arrow_forward
- Explain the experimental procedure of the laboratory preparation of the synthesis of alizarin from anthraquinone Explain the chemistry behind the synthesis of alizarin from anthraquinonearrow_forwardGive the structure of the polyurethane formed by the reaction of toluene diisocyanate with bisphenol A.arrow_forwardQ1: Discuss the molecular basis for the reaction of tollen’s reagent with acetic acid that leads to theformation of silver precipitate.Q2: What are the products of the hydrolysis of ethyl acetate?arrow_forward
- 4 Why must the mixture of (Ethanol, Ethanoic acid and sulphuric acid) not be boiled during esterification process? 5- Write down the type of 2-methyl-2-propanol?arrow_forwardWhat is the critical extent of reaction (pc) based on Carother's approach to gelation for the synthesis of a polyamide using equimolar amounts of diethylene triamine and the acid chloride of trimesic acid (see structures below; assume only the primary amines react). Some helpful equations: Carothers Equation: p. = 2/fav, where fav = (ENfi) /ENi CI H2N `NH2arrow_forwardExplain why the addition of a small amount of glycerol to the polymerization mixture gives a stiffer urethane foam.arrow_forward
- Explain the experimental procedure of the laboratory preparation of the synthesis of sulfonamide from benzene Explain the chemistry behind the synthesis of sulfonamide from benzenearrow_forwardFor the esterification synthesis of isopentyl acetate, acetic acid is one of the component used to prepare the product. why is there a need to add sulfuric acid to the solution if acetic acid is already an acid?arrow_forwardin the esterification of acetylsalicylic acid phosphoric acid was used as a catalyst. Hydrochloric acid is about as strong a mineral acid as sulfuric acid. Why would HCl be not a satisfactory catalyst in this reaction?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole
Seven Name Reactions in One - Palladium Catalysed Reaction (047 - 053); Author: Rasayan Academy - Jagriti Sharma;https://www.youtube.com/watch?v=5HEKTpDFkqI;License: Standard YouTube License, CC-BY