
EP ORGANIC CHEMISTRY-OWL V2 ACCESS
8th Edition
ISBN: 9781305582453
Author: Brown
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 28, Problem 28.10P
Interpretation Introduction
Interpretation:
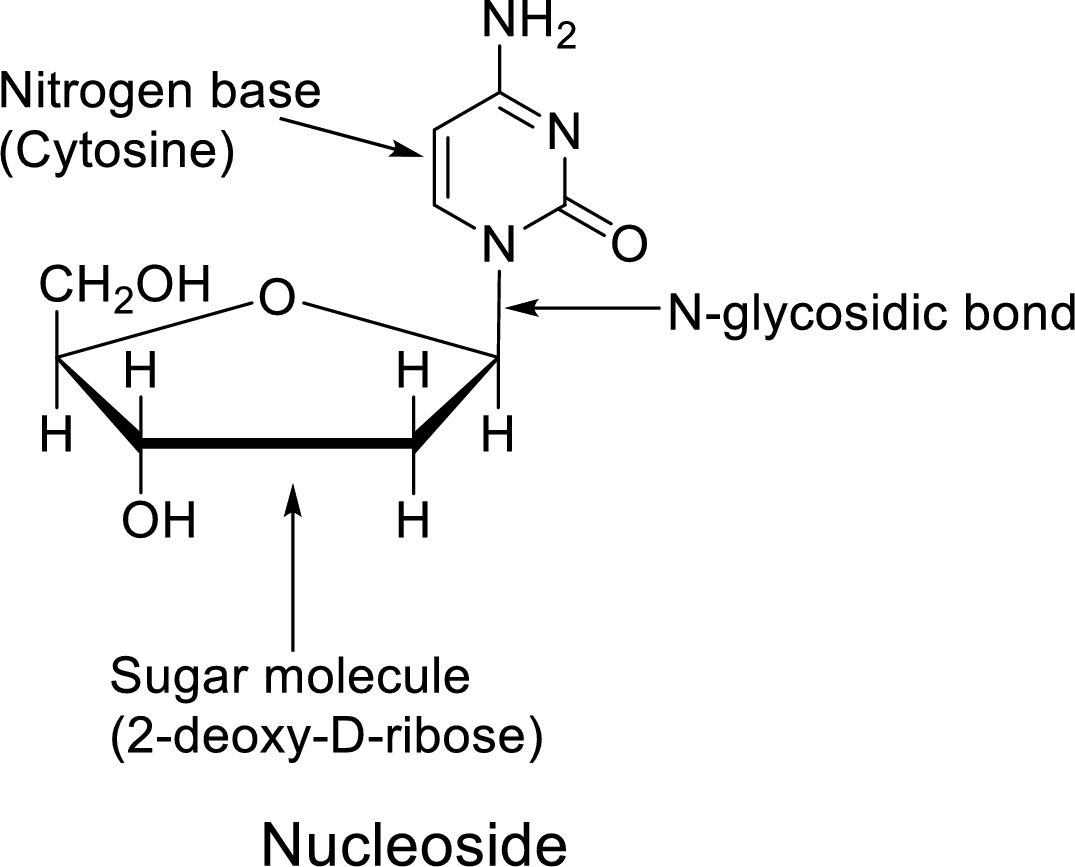
In dilute acid, the glycosidic bond of Nucleoside undergoes hydrolysis to give a pentose and a heterocyclic
Concept Introduction:
The Nucleoside is the component of the
Nucleoside = Nitrogen base + Sugar molecule
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
α-Amino acids can be prepared by treating an aldehyde with ammonia/trace acid, followed by hydrogen cyanide, followed by acid-catalyzed hydrolysis.
Draw the structures of the two intermediates formed in this reaction.
Aspartame is the methyl ester of a dipeptide made of phenylalanine and aspartic acid. what are the expected products of the hydrolysis of aspartame?
Identify the organic functional group and reaction type for the following reaction.
The reactant is a(n)
- carboxylic acid hexose
- Aldohexose
- aldotetrose
-deoxyhexose
-carboxylic acid tetrose
- ketohexose
The product is a(n)
- carboxylic acid tetrose
- aldotetrose
-alcohol hexose
-aldohexose
-carboxylic acid hexose
- alcohol tetrose
The reaction type is
- hemiacetal formation
-hydrolysis
-oxidation( Benedict’s)
-acetal formation
-reduction( hydrogenation)
- mutarotation
Chapter 28 Solutions
EP ORGANIC CHEMISTRY-OWL V2 ACCESS
Ch. 28.1 - Prob. 28.1PCh. 28.2 - Prob. 28.2PCh. 28.2 - Prob. 28.3PCh. 28.3 - Here is a portion of the nucleotide sequence in...Ch. 28.4 - The following section of DNA codes for oxytocin, a...Ch. 28.5 - Prob. 28.6PCh. 28 - Prob. 28.7PCh. 28 - Following are structural formulas for cytosine and...Ch. 28 - Prob. 28.9PCh. 28 - Prob. 28.10P
Ch. 28 - Prob. 28.11PCh. 28 - Prob. 28.12PCh. 28 - Prob. 28.13PCh. 28 - Prob. 28.14PCh. 28 - Prob. 28.15PCh. 28 - Draw a structural formula of the DNA...Ch. 28 - List the postulates of the Watson-Crick model of...Ch. 28 - Prob. 28.18PCh. 28 - Prob. 28.19PCh. 28 - Prob. 28.20PCh. 28 - Prob. 28.21PCh. 28 - Prob. 28.22PCh. 28 - Prob. 28.23PCh. 28 - Prob. 28.24PCh. 28 - Write the DNA complement for 5-ACCGTTAAT-3. Be...Ch. 28 - Prob. 28.26PCh. 28 - Prob. 28.27PCh. 28 - Compare DNA and RNA is these ways. (a)...Ch. 28 - What type of RNA has the shortest lifetime in...Ch. 28 - Prob. 28.30PCh. 28 - Prob. 28.31PCh. 28 - Prob. 28.32PCh. 28 - Write the mRNA codons for the following. (a)...Ch. 28 - Prob. 28.34PCh. 28 - Prob. 28.35PCh. 28 - Prob. 28.36PCh. 28 - Prob. 28.37PCh. 28 - Prob. 28.38PCh. 28 - Prob. 28.39PCh. 28 - What polypeptide is coded for by this mRNA...Ch. 28 - The alpha chain of human hemoglobin has 141 amino...Ch. 28 - Prob. 28.42P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 22-97 Gelatin is derived from collagen by denaturation. Is a gelatin dessert likely to be a good source of dietary protein?arrow_forwardGive the major product that is formed when the primary hydroxyl group of the followingmonosaccharide reacts with propionic anhydride.arrow_forwardWhy can isomaltose be easily hydrolyzed through acid hydrolysis while trehalose cannot undergo acid hydrolysis?arrow_forward
- The hydrolysis of an ester can be sped up by both acidic and basic conditions. Aminolysis of an ester can be sped up by acidic conditions, but not by basic conditions. Explain why.arrow_forwardContrast the structure of glycogen and chitin. Drag the appropriate items to their respective rows. Glycogen Chitin Monomers connect by B-1,4-glycosidic linkages Monomers connect by alpha-1,4-glycosidic linkages Polymers interact with one another via hydrogen bonds Consists of modified glucose residues (NAG) Contains branches arising from alpha-1,6-glycosidic linkages Consists of glucose residuesarrow_forward22-61 Polyglutamic acid (a polypeptide chain made only of glutamic acid residues) has an a-helix conformation below pH 6.0 and a random-coil conformation above pH 6.0. What is the reason for this conformational change?arrow_forward
- 22-42 (a) How many atoms of the peptide bond lie in the same plane? (b) Which atoms are they?arrow_forward22-20 Show how alanine, in solution at its isoelectric point, acts as a buffer (write equations to show why the pH does not change much if we add an acid or a base).arrow_forward22-48 How many amino acid residues in the A chain of insulin are the same in insulin from humans, cattle (bovine), hogs, and sheep?arrow_forward
- 22-104 Why is collagen not a very good source of dietary protein?arrow_forward22-9 What is the difference in structure between tyrosine and phenylalanine?arrow_forward22-47 How many different tetrapeptides can be made (a) if the peptides contain the residues of asparagine, proline, serine, and metbionine and (b) if all 20 amino acids can be used?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning