
Concept explainers
(a)
Interpretation: The Haworth projection for
Concept introduction: The structural representation of sugar molecule in cyclic form is known as Haworth projection. Sugar molecule that has six-membered-ring is known as pyranose and sugar molecule that has five-membered-ring is called furanose.
Answer to Problem 28.45P
The Haworth projection for
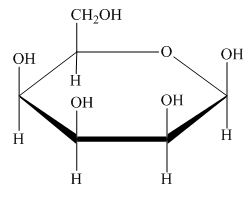
Explanation of Solution
The structure of D-talose is,
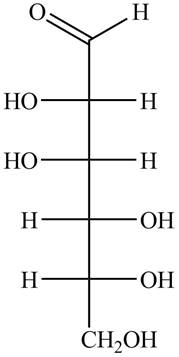
Figure 1
The steps for the conversion of Fischer projection of D-talose into Haworth projection is as follow:
Step-1 Talopyranose ring is formed by the attack of
Step 2 The Haworth projection of D-talopyranose,
Step 3 In the beta-form, the substituents
Step-4 The substituents which are present on the right side in the Fischer projection are drawn on the below the ring in the Haworth projection. Similarly, the substituents which are present on the left side in the Fischer projection are drawn on the above the ring in the Haworth projection.
The conversion of D-talose into
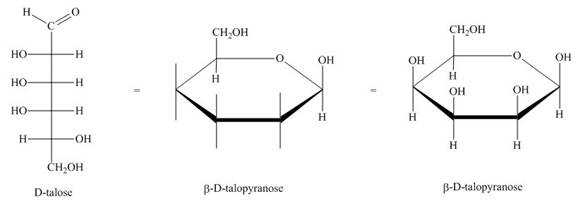
Figure 2
The Haworth projection for
(b)
Interpretation: The Haworth projection for
Concept introduction: The structural representation of sugar molecule in cyclic form is known as Haworth projection. Sugar molecule that has six-membered-ring is known as pyranose and sugar molecule that has five-membered-ring is called furanose.
Answer to Problem 28.45P
The Haworth projection for
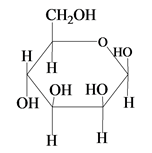
Explanation of Solution
The structure of D-mannose is,
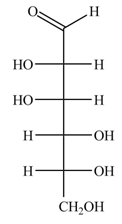
Figure 3
The steps for the conversion of Fischer projection of D-mannose into Haworth projection is as follow:
Step-1 Mannosepyranose ring is formed by the attack of
Step 2 The Haworth projection of D-mannosepyranose,
Step 3 In the beta-form, the substituents
Step-4 The substituents which are present on the right side in the Fischer projection are drawn on the below the ring in the Haworth projection. Similarly, the substituents which are present on the left side in the Fischer projection are drawn on the above the ring in the Haworth projection.
The conversion of D-mannose into D-mannosepyranose is shown below.
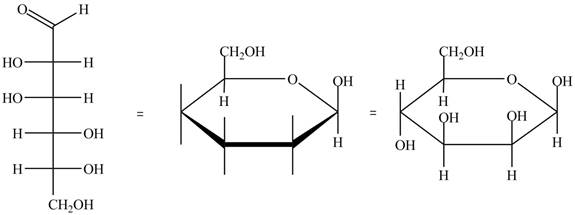
Figure 4
The Haworth projection for
(c)
Interpretation: The Haworth projection for
Concept introduction: The structural representation of sugar molecule in cyclic form is known as Haworth projection. Sugar molecule that has six-membered-ring is known as pyranose and sugar molecule that has five-membered-ring is called furanose.
Answer to Problem 28.45P
The Haworth projection for
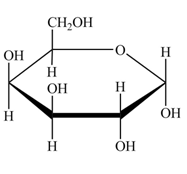
Explanation of Solution
The structure of D-galactose is,
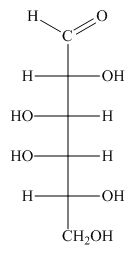
Figure 5
The steps for the conversion of Fischer projection of D-galactose into Haworth projection is as follow:
Step-1 Galactopyranose ring is formed by the attack of
Step 2 The Haworth projection of D-galactopyranose,
Step 3 In the alpha-form, the substituents
Step-4 The substituents which are present on the right side in the Fischer projection are drawn on the below the ring in the Haworth projection. Similarly, the substituents which are present on the left side in the Fischer projection are drawn on the above the ring in the Haworth projection.
The conversion of D-galactose into D-galactopyranose is shown below.
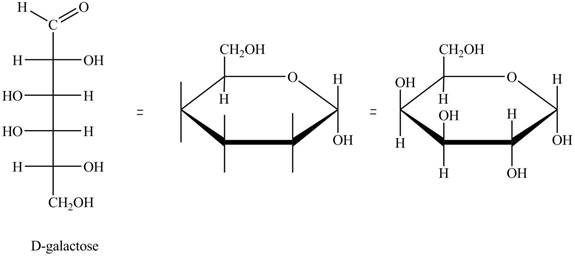
Figure 6
The Haworth projection for
(d)
Interpretation: The Haworth projection for
Concept introduction: The structural representation of sugar molecule in cyclic form is known as Haworth projection. Sugar molecule that has six-membered-ring is known as pyranose and sugar molecule that has five-membered-ring is called furanose.
Answer to Problem 28.45P
The Haworth projection for
Explanation of Solution
The structure of D-ribose is,
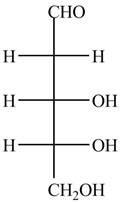
Figure 7
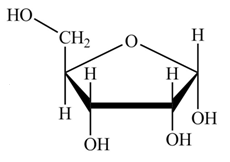
The steps for the conversion of Fischer projection of D-ribose into Haworth projection is as follow:
Step-1 Ribofuranose ring is formed by the attack of
Step 2 The Haworth projection of D-ribofuranose,
Step 3 In the alpha-form, the substituents
Step-4 The substituents which are present on the right side in the Fischer projection are drawn on the below the ring in the Haworth projection. Similarly, the substituents which are present on the left side in the Fischer projection are drawn on the above the ring in the Haworth projection.
The conversion of D-ribose into D-ribofuranose is shown below.
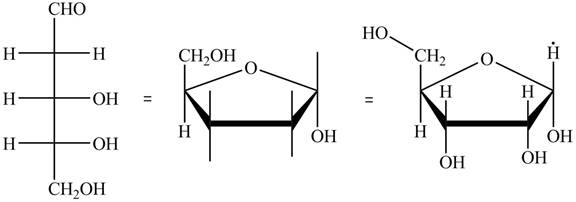
Figure 8
The Haworth projection for
(e)
Interpretation: The Haworth projection for
Concept introduction: The structural representation of sugar molecule in cyclic form is known as Haworth projection. Sugar molecule that has six-membered-ring is known as pyranose and sugar molecule that has five-membered-ring is called furanose.
Answer to Problem 28.45P
The Haworth projection for
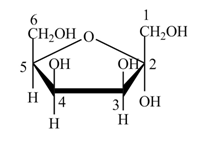
Explanation of Solution
The structure of D-tagatose is,
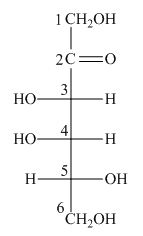
Figure 9
The steps for the conversion of Fischer projection of D-tagatose into Haworth projection is as follow:
Step-1 Tagatofuranose ring is formed by the attack of
Step 2 The Haworth projection of D-tagatofuranose,
Step 3 In the alpha-form, the substituents
Step-4 The substituents which are present on the right side in the Fischer projection are drawn on the below the ring in the Haworth projection. Similarly, the substituents which are present on the left side in the Fischer projection are drawn on the above the ring in the Haworth projection.
The conversion of D-tagatose into D-tagatofuranose is shown below.
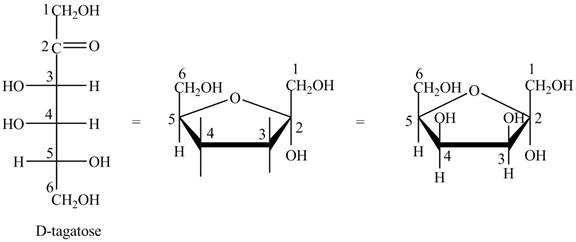
Figure 10
The Haworth projection for
Want to see more full solutions like this?
Chapter 28 Solutions
ALEKS 360 CHEMISTRY ACCESS
- 1mL of Benedict's reagent is added to 1mL of the test solution. The mixture is then heated for 5 minutes in water bath. Appearance of reddish-brown precipitates indicates the presence of a reducing sugar. Glucose and Fructose give a positive result. Sucrose on the other hand gives a negative result. Explain why sucrose give a negative result when subjected to a Benedict's test.arrow_forwardWhich is the more effective buffer at physiological pH, a solution of 0.1 M glycylglycylglycylglycine or a solution of 0.2 M glycine?arrow_forwardWhat is the Fisher Projection, Haworth representation and Pyranose/furanoseRing structures of β-D-talopyranose and α-D-xylulose?arrow_forward
- what is Edman’s degradation? How will you break disulfide bonds in proteins?arrow_forwardWhich of the following combination in each pair is likely to produce more Maillard browning when heated at 95 °C for 4 hours? Explain the chemical basis for your choice. Sucrose + glycine, pH 7.0 vs. glucose + glycine, pH 7.0 Maltose with a dextrose equivalency (DE) of 20 + glycine, pH 9.0 vs. maltose + glycine, pH 9.0 Lactose + glycine, pH 8.0 vs. lactose + glycine, pH 4.0arrow_forwardTreatment of a 258 mg sample of amylopectin by the methylation and hydrolysis procedure described yielded 12.4 mg of 2,3‑di‑O‑methylglucose. Determine what percentage of the glucose residues in amylopectin contained an (α1→6) branch. (Assume that the average molecular weight of a glucose residue in amylopectin is 162 g/mol and the molecular weight of 2,3‑di‑O‑methylglucose is 208 g/mol.) ( α1→6) branched glucose residues: %arrow_forward
- Which control test tubes contained reducing sugars? Are these the results consistent with the sugars tested, explain? Sucrose and lactose are both disaccharides, explain why the test results are the same or different? Sucrose 8 minutes Blue color like Benedict's solution, not reaction. - Lactose 8 minutes orange-red color + Glucose 8 minutes Orange-red color +arrow_forwardi would like to ask the following questions: 1. Aside from the reagent and the positive results, what is the difference between Fehling’s Test, Moore’s test and Benedict’s Test if they are all used to detect the presence of reducing sugar?2. In Benedict’s Test, why are there different colors? Why not just one color in varying shades?3. Why is sucrose not a reducing sugar even though most disaccharides are reducing sugars?arrow_forwardTrue or False 1. Mutarotation affects the reducing property of carbohydrates. 2. Anthrone test can be use to test for the helical configuration of a polysaccharide. 3. Upon treatment with phenylhydrazine reagent & hydrolysis, maltose gives glucuronic acid and glucosazone.arrow_forward
- Chemical Connections 20D states that the antigen in the red blood cells of a person with B-type blood is a galactose unit. Show schematically how the antibody of a person with A-type blood would aggregate the red blood cells of a B-type person if such a transfusion were made by mistake.arrow_forwardexpanded structure of the D-tagatose Fischer projection of D-tagatose complete steps of Haworth projection of D-tagatosearrow_forwardWhat is a reducing sugar? What other types of sugars besides glucose might you measure using the dinitrosalicylic acid (DNS) reagent?arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

