
ORGANIC CHEMISTRY W/ALEKS
6th Edition
ISBN: 9781264905430
Author: SMITH
Publisher: MCG CUSTOM
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 28, Problem 58P
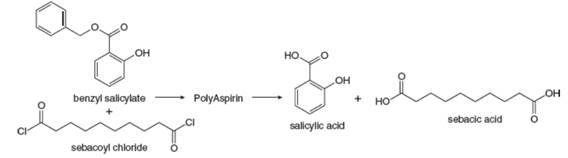
Researchers at Rutgers University have developed biocompatible
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Another source of phosphorus in water is known as organic phosphorus which commonly finds its way into natural bodies
of water from human and animal waste and food residues.
If you wish to measure the phosphorus content due to organic matter in the water sample using the
vanadomolybdophosphoric acid method, you must first "digest" the sample to convert the organic phosphorus to
orthophosphate.
O True
O False
Researchers at Rutgers University have developed biocompatible polymers that degrade into nonsteroidal anti-inflammatory drugs. For example, the reaction of two equivalents of benzyl salicylate and one equivalent of sebacoyl chloride forms a poly(anhydride ester) called PolyAspirin, which hydrolyzes to salicylic acid (an anti-inflammatory agent) and sebacic acid, which is excreted. This technology can perhaps be used for localized drug delivery at specific sites of injury. What is the structure of PolyAspirin?
What is the structural difference between HDP and LDP? How does the structure account for different behaviour and nature, hence the use of a polymer?
Chapter 28 Solutions
ORGANIC CHEMISTRY W/ALEKS
Ch. 28.1 - Prob. 1PCh. 28.2 - Prob. 2PCh. 28.2 - Prob. 3PCh. 28.2 - Draw the mechanism for the radical polymerization...Ch. 28.2 - Prob. 8PCh. 28.2 - Prob. 9PCh. 28.2 - Prob. 10PCh. 28.3 - Problem 30.12
What polymer is formed by anionic...Ch. 28.5 - Prob. 12PCh. 28.5 - Prob. 13P
Ch. 28.6 - Problem 30.15
What polyamide is formed from each...Ch. 28.6 - Prob. 19PCh. 28.7 - Prob. 20PCh. 28.8 - Prob. 21PCh. 28.9 - Prob. 22PCh. 28.9 - Prob. 23PCh. 28 - Prob. 24PCh. 28 - Prob. 25PCh. 28 - 30.30 Draw each polymer in Problem 30.29 using the...Ch. 28 - Prob. 44PCh. 28 - 30.49 Draw the products of each reaction.
a. e....Ch. 28 - Prob. 56PCh. 28 - 30.56 Compound A is a novel poly (ester amide)...Ch. 28 - 30.57 Researchers at Rutgers University have...Ch. 28 - 30.58 Melmac, a thermosetting polymer formed from...Ch. 28 - 30.59 Although chain branching in radical...Ch. 28 - Prob. 61P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- PTT (polytrimethylene terephthalate) is a polyester formed from 1,3-propanediol (HOCH 2CH 2CH 2OH) by the given reaction. PTT is sold under the trade name Sorona by DuPont. 1,3-Propanediol can now be made from a plant source like corn, rather than from petroleum starting materials. This makes PTT a more environmentally friendly polyester than PET. What is the structure of PTT?arrow_forwardThe plastic known as PETE (polyethyleneterephthalate) is used to make plastic soft drink bottles and containers for salad dressing, shampoos, and dishwashing liquids. PETE is a polymer of terephthalic acid and ethylene glycol. Today, PETE is the most widely recycled of all the plastics. After it is separated from other plastics, PETE can be used in polyester fabric, door mats, and tennis ball containers. In 2015, 1.84×109 lb1.84×109 lb of PETE bottles were recycled in the U.S. The density of PETE is 1.38 g/mLg/mL. How many kilograms of PETE bottles were recycled in 2015 in the U.S.? Express your answer to three significant figures.arrow_forwardThe plastic known as PETE (polyethyleneterephthalate) is used to make plastic soft drink bottles and containers for salad dressing, shampoos, and dishwashing liquids. PETE is a polymer of terephthalic acid and ethylene glycol. Today, PETE is the most widely recycled of all the plastics. After it is separated from other plastics, PETE can be used in polyester fabric, door mats, and tennis ball containers. In 2015, 1.84×109 lb1.84×109 lb of PETE bottles were recycled in the U.S. The density of PETE is 1.38 g/mLg/mL. What volume, in liters, of PETE bottles were recycled in 2015 in the U.S.? Express your answer to three significant figures.arrow_forward
- The plastic known as PETE (polyethyleneterephthalate) is used to make plastic soft drink bottles and containers for salad dressing, shampoos, and dishwashing liquids. PETE is a polymer of terephthalic acid and ethylene glycol. Today, PETE is the most widely recycled of all the plastics. After it is separated from other plastics, PETE can be used in polyester fabric, door mats, and tennis ball containers. In 2015, 1.84×109 lb1.84×109 lb of PETE bottles were recycled in the U.S. The density of PETE is 1.38 g/mLg/mL. Suppose a landfill holds 2.6×107 L2.6×107 L of recycled PETE. If all the PETE bottles recycled in 2015 in the U.S. were placed instead in landfills, how many landfills would be needed? Express your answer as an integer.arrow_forwardCan you explain condensation polymerization and give an example with structure of one with 2 carboxylic acid groups and 2 OH groups.arrow_forwardcan you explain to me in details the manufacturing process of polystyrene ?arrow_forward
- In reference to cloth or fiber, the term acetate usually means cellulose acetate, a semisynthetic polymer made by treating cellulose with acetic anhydride. Cellulose acetate is spun into yarn by dissolving it in acetone or methylene chloride and forcing the solution through spinnerets into warm air, where the solvent evaporates. Predict what usually happens when students wear polyvinyl chloride shoes to the organic laboratory.arrow_forwardThe most common industrial application of diethyl phthalate (or more generally, phthalate esters) is as a plasticizer. What is a plasticizer? Explain why plastics become brittle over time.arrow_forwardPoly(ethylene terephthalate), or PET, is a polyester used to make soft-drink bottles. It is prepared by reaction of ethylene glycol with 1,4-benzenedicarboxylic acid (terephthalic acid). Draw the structure of PET.arrow_forward
- Give an example of an ester polymer and cite its usearrow_forwardIn reference to cloth or fiber, the term acetate usually means cellulose acetate, a semisynthetic polymer made by treating cellulose with acetic anhydride. Cellulose acetate is spun into yarn by dissolving it in acetone or methylene chloride and forcing the solution through spinnerets into warm air, where the solvent evaporates. An organic chemistry student wore a long-sleeved acetate blouse to the laboratory. She was rinsing a warm separatory funnel with acetone when the pressure rose and blew out the stopper. Her right arm was drenched with acetone, but she was unconcerned because acetone is not very toxic. About ten minutes later, the right arm of the student’s blouse disintegrated into a pile of white fluff, leaving her with a ragged short sleeve and the tatters of a cuff remaining around her wrist. Explain how a substance as innocuous as acetone ruined the student’s blouse.arrow_forwardWrite the chemical structure of a non-biodegradable polymer (Polystyrene) and explain (in one line), based on it's chemical structure why it has this propertyarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning
Molecular spectroscopy; Author: Vidya-mitra;https://www.youtube.com/watch?v=G6HjLIWvCQo;License: Standard YouTube License, CC-BY