
ORGANIC CHEMISTRY (LL+SM+ACCESS)
6th Edition
ISBN: 9781264309436
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 28, Problem 60P
Although chain branching in radical
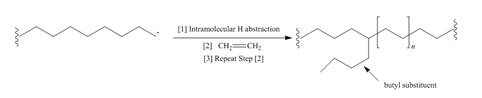
a. Draw a stepwise mechanism that illustrates which H must be intramolecularly abstracted to form butyl substituents.
b. Suggest a reason why the abstraction of this H is more facile than the abstraction of other H’s.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Which of the following molecules can be used as a monomer to generate this polymer?
So the answer's B... im having quite the trouble
understanding where in the polymer the
double bond broke. Could someone explain
and draw in EXTREME details how the
answer's B and not any other answer choice?
1.- How does the molecular weight change vs % conversion in a step polymerization? Explain this behavior and why does it occur?
2.- Name at least 3 requirements for obtaining a high molecular weight polymer by step polymerization.
3.- How can you minimize cyclization reactions in a step polymerization? Explain with equations and narrative Why do you want to minimize cyclization? Is it possible to eliminate cyclic products completely?
Which polymer do you think has the higher Tg, Kevlar (see Solved Problem 26.11) or the polyphthalamide described in Problem 26.12 (p. 1328)? Explain your reasoning.
Chapter 28 Solutions
ORGANIC CHEMISTRY (LL+SM+ACCESS)
Ch. 28.1 - Prob. 1PCh. 28.2 - Prob. 2PCh. 28.2 - Prob. 3PCh. 28.2 - Draw the mechanism for the radical polymerization...Ch. 28.2 - Prob. 8PCh. 28.2 - Prob. 9PCh. 28.2 - Prob. 10PCh. 28.3 - Problem 30.12
What polymer is formed by anionic...Ch. 28.5 - Prob. 12PCh. 28.5 - Prob. 13P
Ch. 28.6 - Problem 30.15
What polyamide is formed from each...Ch. 28.6 - Prob. 19PCh. 28.7 - Prob. 20PCh. 28.8 - Prob. 21PCh. 28.9 - Prob. 22PCh. 28.9 - Prob. 23PCh. 28 - Prob. 24PCh. 28 - Prob. 25PCh. 28 - 30.30 Draw each polymer in Problem 30.29 using the...Ch. 28 - Prob. 44PCh. 28 - 30.49 Draw the products of each reaction.
a. e....Ch. 28 - Prob. 56PCh. 28 - 30.56 Compound A is a novel poly (ester amide)...Ch. 28 - 30.57 Researchers at Rutgers University have...Ch. 28 - 30.58 Melmac, a thermosetting polymer formed from...Ch. 28 - 30.59 Although chain branching in radical...Ch. 28 - Prob. 61P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Rank the following monomers in order of increasing reactivity toward anionic polymerization (least reactive to most reactive).arrow_forwardWhat products are formed by ring-opening metathesis polymerization of each alkene? a. b. C.arrow_forwardContrast a single and a double bond with regard to:a. distance of separation of the bonded nucleib. strength of the bondarrow_forward
- 2) For the monomer given determine what type of polymerization (radical, anionic, cationic, and/or step-wise) it will undergo most readily or if it will not undergo polymerization at all. Explain your answer. A.arrow_forwardRank the following monomers in order of increasing reactivity toward cationic polymerization (least reactive to most reactive).arrow_forwardWhat polymer would form folowing the following reaction? Run A B C. CompsundAarrow_forward
- Which polymer would be more flexible at room temperature poly(methyl acrylate) or poly(methyl methylcrylate)? Why? Support and explain answerarrow_forwardCationic polymerization of 3-phenylpropene (CH2=CHCH2Ph) affords Aas the major product rather than B. Draw a stepwise mechanism toaccount for this observation.arrow_forward(a) Show how poly(propylene oxide) can be synthesized via anionic ring-opening polymerization. (b) Draw the mechanism for the initiation and first two propagation steps.arrow_forward
- For indicated reaction provide: Ionic polymerization of propene. a. general equationb. Mechanismc. Discussion of regioselectivityd. Discussion of the proportion between the products formed (if more than one is formed).e. Discussion of stereoselectivity.arrow_forwardSelect the appropriate synthetic route to convert methylcyclobutane into cyclopentene. ?? 1 1. KOtBu; 2. BH3-THF; 3. H₂O2, NaOH; 4: H₂SO4, heat 1. H₂, Lindlar's catalyst; 2. BH3-THF; 3. H₂O2, NaOH 1. Br₂, hv; 2. KOtBu; 3. BH3-THF; 4. H₂O2, NaOH; 5: H₂SO4, heat 1.BH3-THF; 2. H₂O2, NaOH; 3. H₂, Pt 1. Br₂, hv; 2. KOtBu; 3. H₂O₂, NaOH; 4: H₂SO4, heatarrow_forward(d) show termination and propogation process in polyethylene terephthalate (PET).arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
CBSE Class 12 Chemistry || Polymers || Full Chapter || By Shiksha House; Author: Best for NEET;https://www.youtube.com/watch?v=OxdJlS0xZ0Y;License: Standard YouTube License, CC-BY