
Concept explainers
What is the predominant form of each of the following amino acids at
(a)
Interpretation: The predominant form of valine at
Concept introduction: At isoelectric point, the amino acids exist in their neutral form. The amine groups exists as
Answer to Problem 29.37P
The predominant form of valine at
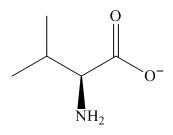
The overall charge on it is
Explanation of Solution
At isoelectric point, the amino acids exist in their neutral form. The amine groups exists as
The isoelectric point of valine is

Figure 1
The overall charge on valine at
The predominant form of valine at
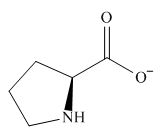
The overall charge on it is
(b)
Interpretation: The predominant form of proline at
Concept introduction: At isoelectric point, the amino acids exist in their neutral form. The amine groups exists as
Answer to Problem 29.37P
The predominant form of proline at
Explanation of Solution
At isoelectric point, the amino acids exist in their neutral form. The amine groups exists as
The isoelectric point of proline is

Figure 2
The overall charge on proline at
The predominant form of proline at
(c)
Interpretation: The predominant form of glutamic acid at
Concept introduction: At isoelectric point, the amino acids exist in their neutral form. The amine groups exists as
Answer to Problem 29.37P
The predominant form of glutamic acid at
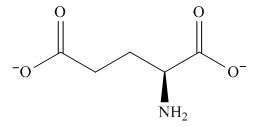
The overall charge on it is
Explanation of Solution
At isoelectric point, the amino acids exist in their neutral form. The amine groups exists as
The isoelectric point of glutamic acid is

Figure 3
The overall charge on glutamic acid at
The predominant form of glutamic acid at
The overall charge on it is
(d)
Interpretation: The predominant form of lysine at
Concept introduction: At isoelectric point, the amino acids exist in their neutral form. The amine groups exists as
Answer to Problem 29.37P
The predominant form of lysine at

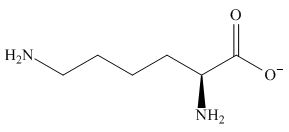
The overall charge on it is
Explanation of Solution
At isoelectric point, the amino acids exist in their neutral form. The amine groups exists as
The isoelectric point of lysine is

Figure 4
The overall charge on lysine at
The predominant form of lysine at
Want to see more full solutions like this?
Chapter 29 Solutions
ORG.CHEMISTRY W/ACCESS+MODEL KIT PKG
- The -helical parts of myoglobin and other proteins stop whenever a proline residue is encountered in the chain. Why is proline never present in a protein helix?arrow_forward4b) Canavanine is closely related to arginine, and like arginine its side group has a +1 charge when protonated. If you dissolved canavanine in an aqueous solution at pH 10, what would the net charge on a molecule of canavanine be? Please show your work or make it clear how you determined the charge contribution from each ionizable group.arrow_forwardIf the pH of a solution of Arginine is equal to a half of the 3 pka values of all of its functional groups, what will be the charge on the majority of the Arg molecules in that solution? Please explain. Am i supposedd to add up the three pka values and then divide it in half to find the pH? Thanksarrow_forward
- Which of the following best represents the structure of an amino acid in basic solution (pH=11)?arrow_forwardAn amino acid residue side chain is deprontonated at pH9.5. What is the pKa of this residue if it is fully deprotonated at this pH?arrow_forwardExplain why the pI of lysine is the average of the pKa values of its two protonated amino groups.arrow_forward
- There are two pH values in which Glutamic acid will have an a complete net charge of 2.17? what is the lowest PH1 and highest of pH2 round your amswers to the nearest 0.1 no orher information was providedarrow_forwardUsing the information below, determine the amino acid sequence of the peptide, and explain how your structure is consistent with each piece of information. Complete hydrolysis by 6 M HCl at 110°C followed by amino acid analysis indicated the presence of Gly, Leu, Phe, and Tyr in a 2:1:1:1 molar ratio. Treatment of the peptide with1-fluoro-2,4-dinitrobenzene followed by complete hydrolysis and chromatography indicated the presence of the 2,4-dinitrophenyl derivative of tyrosine. No free tyrosine could be found. Complete digestion of he peptide with pepsin (which cleaves on the amino side of aromatic residues) followed by chromatography yielded a dipeptide containing Phe and Leu and a tripeptide containing Tyr and Gly in a 1:2 ratio.arrow_forwardAlmost all proteins are composed from a set of about _____ amino acids.a. 4b. 10c. 20d. 50 ______ amino acids are considered essential.a. 5b. 10c. 15d. 20 An amino acid that has an indole group side chain.a. Trpb. Phec. Tyrd. ProWhich of the following is the simplest amino acid?a. Alab. Glyc. Vald. ProWhich of these will not give a blue solution with Ninhydrin testa. Prob. Phec. Alad. CysA neutral molecule with a positive and negative electrical charge.a. Isoelectric pointb. Zwitterionsc. Bufferd. Amphoteric3 amino acids joined together by two peptide bonds.a. Dipeptideb. Tripeptidec. Tetrapeptided. PolypeptideAn amino acid that can form disulfide bondsa. Cysteineb. Methioninec. Cystined. SerineWhich of the following is not a property of amino acidsa. Bufferb. Isoelectric pointc. Zwitterionsd. Insoluble in waterThe protein primary structure is held together bya. Hydrogen bondsb. Disulfide bondsc. Peptide bondsd. Protein bondsarrow_forward
- Finally, you want to make completely certain that you purified the correct protein and not some other protein (like biochemisnotfunase). To confirm the identity of your protein, you run a Western blot. When doing a Western, one typically needs a solution of phosphate-buffered saline, which is comprised of 10 mM phosphate buffer(pH 8.0) and 0.9% (w/v) NaCl. Describe how you would make 500 mL of this solution including the grams of sodium phosphate, monobasic and sodium phosphate, dibasic that you would need. Making the phosphate buffer is a little tricky. Be sure to use the correct ratio of monobasic and dibasic sodium phosphate to get the correct pH. The Henderson- Hasselbalch equation might come in handy here. Finally, the molecular weight of sodium phosphate, monobasic (NaH2PO4) is 119.98 g/mol and the molecular weight of sodium phosphate, dibasic (Na2HPO4) is 141.96 g/mol.arrow_forwardQ1)In the pH range 1.82 to 8.99, H2Arg+ is the principal form of arginine. Which is the second most prominent species at pH 6.0? At pH 5.0?Q2) (a) Draw the structure of the predominant form (principal species) of 1,3-dihydroxybenzene at pH 9.00 and at pH 11.00.(b) What is the second most prominent species at each pH?(c) Calculate the percentage in the major form at each pHarrow_forwardWhere is the effective buffering range for this amino acid in the acidic region?arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning


