
Concept explainers
(a)
Interpretation:
The given condition should be classified whether as a cause of respiratory or metabolic acidosis or alkalosis using the table 29.4.
Concept introduction:
The highly specific acid-base quality of various body fluid remains regulated within narrow limits because of high sensitivity of
Alkalosis is a term which refers to an increase in pH above the normal average of
Acidosis is a term which refers to an increase in
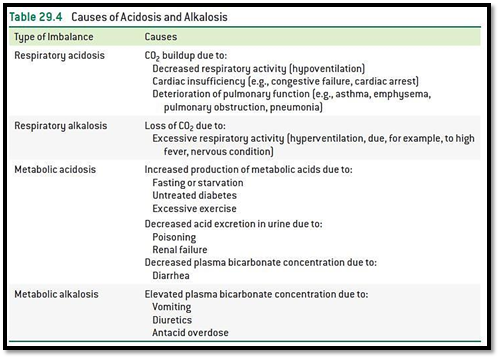
Figure 1
(b)
Interpretation:
The given condition should be classified whether as a cause of respiratory or metabolic acidosis or alkalosis using the table 29.4.
Concept introduction:
The highly specific acid-base quality of various body fluid remains regulated within narrow limits because of high sensitivity of metabolism to the
Alkalosis is a term which refers to an increase in pH above the normal average of
Acidosis is a term which refers to an increase in

Figure 1
(c)
Interpretation:
The given condition should be classified whether as a cause of respiratory or metabolic acidosis or alkalosis using the table 29.4.
Concept introduction:
The highly specific acid-base quality of various body fluid remains regulated within narrow limits because of high sensitivity of metabolism to the
Alkalosis is a term which refers to an increase in pH above the normal average of
Acidosis is a term which refers to an increase in

Figure 1
Want to see the full answer?
Check out a sample textbook solution
Chapter 29 Solutions
FUND.OF GEN CHEM CHAP 1-13 W/ACCESS
- Describe the control of blood carbonic acid levels through the respiratory system.arrow_forwardDescribe the conservation of bicarbonate ions in the renal system.arrow_forwardWhich of the following statements is true for Bohr effect: a. Bohr effect describes carbon monoxide poisoning. b. Bohr effect prevents oxygen binding to hemoglobin. c. Bohr effect allows effective oxygen discharge in tissues due to higher acidity (decreased pH) than in lungs. d. Bohr effect allows effective oxygen discharge in tissues due to lower acidity (increased pH) than in lungs.arrow_forward
- Metabolic alkalosis refers to a process whereupon decreased hydrogen ion, or increased bicarbonate, concentrations occur in the body, and the blood pH becomes too basic. Answer the following questions: How does the body regain its acid-base equilibria/balance? What happens when that balance does not happen? Will a buffer system still work under these conditions?arrow_forwardhow does the process of exhalation influence the pH of blood, considering the role of carbon dioxide (CO2) removal in regulating acid-base balance? Discuss the physiological mechanisms involved in maintaining blood pH during exhalation, including the role of respiratory control centers and the relationship between CO2 levels, bicarbonate, (HCO3-), carbonic acid (H2CO3) formation, and pH changes in the bloodstreamarrow_forwardWhich reaction does the enzyme carbonic anhydrase catalyze?arrow_forward
 Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning
Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning
Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College
Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College




