
Draw the four water molecules that can hydrogen-bond to this water molecule. Label the bonds and the partial negative and positive charges that account for the formation of these hydrogen bonds.
To draw: The four water molecules that can form hydrogen-bond to the given water molecule and label the bonds and the partial negative and positive charges that account for the formation of these hydrogen bonds.
Introduction: A water molecule is shaped like a ‘V’ that consists of an oxygen atom and two hydrogen atoms. Oxygen is more electronegative than hydrogen. Thus, the shared electrons in the O-H covalent bond are more attracted by the oxygen atom.
Answer to Problem 1IQ
Pictorial representation: Fig.1 represents the four water molecules that can form hydrogen-bond for the given water molecule and label the bonds and the partial negative and positive charges that account for the formation of these hydrogen bonds.
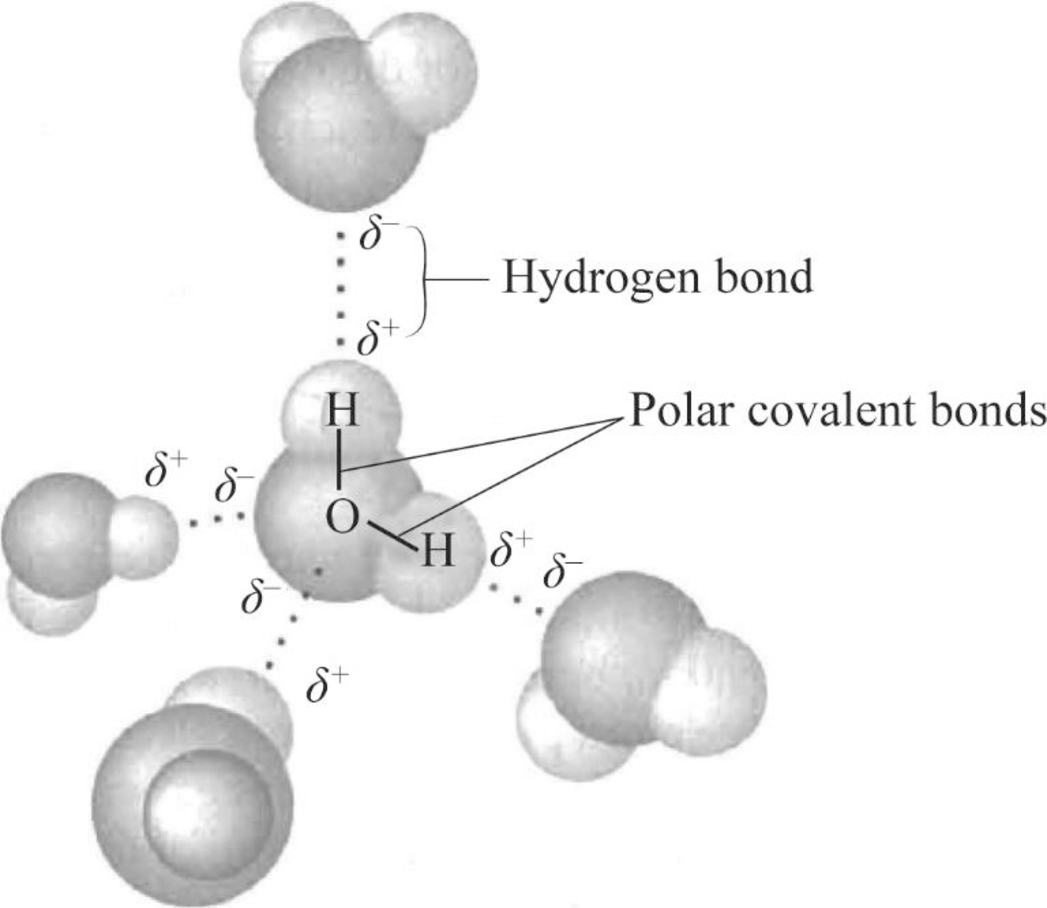
Fig.1: Four water molecules bind with one water molecule
Explanation of Solution
Electronegativity is the tendency of an atom in a molecule to attract electrons shared in a covalent bond. If one atom is more electronegative, the electrons would be more on the side of that atom. Oxygen is more electronegative than hydrogen. In a water molecule, there are two polar covalent bonds between oxygen and hydrogen. Hence, there are two regions of partial negative charge on oxygen and a partial positive charge on each hydrogen atom.
The partial negatively charged oxygen atom of a water molecule is attracted to the partial positively charged hydrogen of the other water molecule. As per Fig.1, atoms on the partial charged regions can bind to oppositely charged regions on other adjoining water molecules. There are four regions of partial charge in a water molecule. Thus, the central water molecule can form a hydrogen bonds with four other water molecules.
Want to see more full solutions like this?
Chapter 3 Solutions
Biology - Study Guide
- Name three vital properties of water in living cells.arrow_forwardWhich type of bond represents a weak chemical bond? a. hydrogen bond b. ionic bond c. covalent bond d. polar covalent bondarrow_forwardIn ________ reactions, small molecules are linked by covalent bonds, and water can also form. a. hydrophilic b. hydrolysis c. condensation d. ionicarrow_forward
- Indicate whether the statement below is true or false.Any covalently bonded H atom can participate in a hydrogen bond if it comes in close proximity with an oxygen atom that forms part of a water molecule.arrow_forwardUsing appropriate examples, explain how hydrogen bonding givesrise to three properties of water that are essential to life.arrow_forwardThis occurs when the cations and anions are attracted to the positive and negative ends of water molecules: A. ionic bonding B. dissociation C. covalent bonding D. hydrogen bondingarrow_forward
- Which of the following elements is or are common to both lipids and proteins? A. Carbon only B. Both hydrogen and oxygen C. Sulphur only D. Sulphur and nitrogenarrow_forwardWhich of the following statements is true? a. Acids and bases cannot mix together. b. Acids and bases will neutralize each other. c. Acids, but not bases, can change the pH of a solution. d. Acids donate hydroxide ions (OH–); bases donate hydrogen ions (H+).arrow_forwardThe type of bonding that happens between atoms within a water molecule is which of the following? a. Polar covalent bonding b.ionic bonding c. Non polar covalent bonding d. Hydrogen bondingarrow_forward
- In a water molecule, hydrogens are partially _____; oxygens are partially _____.arrow_forwardWhich pH value indicates a solution that has more hydrogen ions that hydroxide ions? pH 9 pH 4 pH 7arrow_forwardWhich of the following is an example of a hydrogen bond? the bond between the H of one water molecule and the O of another water molecule the bond between C and H in methane the bond between Na and Cl in salt the bond between Mg and Cl in MgCl2 the bond between two hydrogen atomsarrow_forward
 Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning
Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College
Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College

