
Determine the number of unpaired electrons in each of the following atoms in the ground state and identify each as diamagnetic or paramagnetic: (a) C, (b) S, (c) Cu, (d) Pb, (e) Ti.
(a)
Interpretation:
The number of unpaired electrons in the given atoms with its diamagnetic or paramagnetic behaviour should be given by knowing their ground-state electron configurations.
Concept Introduction:
An orbital is an area of space in which electrons are orderly filled. The maximum capacity in any type of orbital is two electrons. An atomic orbital is defined as the region of space in which the probability of finding the electrons is highest. It is subdivided into four orbitals such as
There are three basic principles in which orbitals are filled by the electrons.
- 1. Aufbau principle: In German, the word 'aufbau' means 'building up'. The electrons are arranged in various orbitals in the order of increasing energies.
- 2. Pauli exclusion principle: An electron does not have all the four quantum numbers.
- 3. Hund’s rule: Each orbital is singly engaged with one electron having the maximum same spin capacity after that only pairing occurs.
The electron configuration is the allocation of electrons of an atom in atomic orbitals. Electronic configuration of a particular atom is written by following the three basic principles. If all the atomic orbitals are filled by electrons, then the atom is diamagnetic in nature. Diamagnetic atoms are repelled by the magnetic field. If one or more unpaired electrons are present in an atom, then that atom is paramagnetic in nature. Paramagnetic atoms are attracted to the magnetic field.
To find: Count the number of unpaired electrons in
Answer to Problem 3.104QP
The number of unpaired electrons in
Explanation of Solution
Carbon (
The noble gas core for
Put all the electrons in the atomic orbitals by following Aufbau principle, Pauli exclusion principle and Hund’s rule.
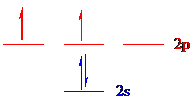
The 4 electrons of carbon (
The unpaired electrons are present in
(b)
Interpretation:
The number of unpaired electrons in the given atoms with its diamagnetic or paramagnetic behavior should be given by knowing their ground-state electron configurations.
Concept Introduction:
An orbital is an area of space in which electrons are orderly filled. The maximum capacity in any type of orbital is two electrons. An atomic orbital is defined as the region of space in which the probability of finding the electrons is highest. It is subdivided into four orbitals such as
There are three basic principles in which orbitals are filled by the electrons.
- 1. Aufbau principle: In German, the word 'aufbau' means 'building up'. The electrons are arranged in various orbitals in the order of increasing energies.
- 2. Pauli exclusion principle: An electron does not have all the four quantum numbers.
- 3. Hund’s rule: Each orbital is singly engaged with one electron having the maximum same spin capacity after that only pairing occurs.
The electron configuration is the allocation of electrons of an atom in atomic orbitals. Electronic configuration of a particular atom is written by following the three basic principles. If all the atomic orbitals are filled by electrons, then the atom is diamagnetic in nature. Diamagnetic atoms are repelled by the magnetic field. If one or more unpaired electrons are present in an atom, then that atom is paramagnetic in nature. Paramagnetic atoms are attracted to the magnetic field.
To find: Count the number of unpaired electrons in
Answer to Problem 3.104QP
The number of unpaired electron in
Explanation of Solution
The noble gas core for
Put all the electrons in the atomic orbitals by following Aufbau principle, Pauli exclusion principle and Hund’s rule.
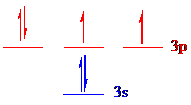
All the 6 electrons of
The unpaired electrons are present in
(c)
Interpretation:
The number of unpaired electrons in the given atoms with its diamagnetic or paramagnetic behavior should be given by knowing their ground-state electron configurations.
Concept Introduction:
An orbital is an area of space in which electrons are orderly filled. The maximum capacity in any type of orbital is two electrons. An atomic orbital is defined as the region of space in which the probability of finding the electrons is highest. It is subdivided into four orbitals such as
There are three basic principles in which orbitals are filled by the electrons.
- 1. Aufbau principle: In German, the word 'aufbau' means 'building up'. The electrons are arranged in various orbitals in the order of increasing energies.
- 2. Pauli exclusion principle: An electron does not have all the four quantum numbers.
- 3. Hund’s rule: Each orbital is singly engaged with one electron having the maximum same spin capacity after that only pairing occurs.
The electron configuration is the allocation of electrons of an atom in atomic orbitals. Electronic configuration of a particular atom is written by following the three basic principles. If all the atomic orbitals are filled by electrons, then the atom is diamagnetic in nature. Diamagnetic atoms are repelled by the magnetic field. If one or more unpaired electrons are present in an atom, then that atom is paramagnetic in nature. Paramagnetic atoms are attracted to the magnetic field.
To find: Count the number of unpaired electrons in
Answer to Problem 3.104QP
The number of unpaired electron in
Explanation of Solution
The noble gas core for
Put all the 11 electrons in the atomic orbitals by following Aufbau principle, Pauli exclusion principle and Hund’s rule.
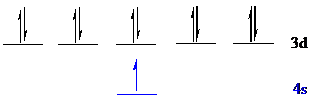
All the 11 electrons of
The unpaired electron is present in
(d)
Interpretation:
The number of unpaired electrons in the given atoms with its diamagnetic or paramagnetic behavior should be given by knowing their ground-state electron configurations.
Concept Introduction:
An orbital is an area of space in which electrons are orderly filled. The maximum capacity in any type of orbital is two electrons. An atomic orbital is defined as the region of space in which the probability of finding the electrons is highest. It is subdivided into four orbitals such as
There are three basic principles in which orbitals are filled by the electrons.
- 1. Aufbau principle: In German, the word 'aufbau' means 'building up'. The electrons are arranged in various orbitals in the order of increasing energies.
- 2. Pauli exclusion principle: An electron does not have all the four quantum numbers.
- 3. Hund’s rule: Each orbital is singly engaged with one electron having the maximum same spin capacity after that only pairing occurs.
The electron configuration is the allocation of electrons of an atom in atomic orbitals. Electronic configuration of a particular atom is written by following the three basic principles. If all the atomic orbitals are filled by electrons, then the atom is diamagnetic in nature. Diamagnetic atoms are repelled by the magnetic field. If one or more unpaired electrons are present in an atom, then that atom is paramagnetic in nature. Paramagnetic atoms are attracted to the magnetic field.
To find: Count the number of unpaired electrons in
Answer to Problem 3.104QP
The number of unpaired electron in
Explanation of Solution
The noble gas core for
Put all the 28 electrons in the atomic orbitals by following Aufbau principle, Pauli exclusion principle and Hund’s rule.
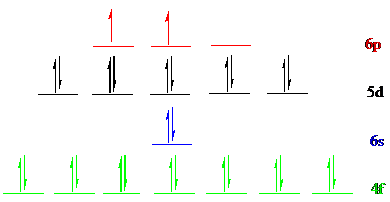
All the 28 electrons of
The unpaired electrons are present in
(e)
Interpretation:
The number of unpaired electrons in the given atoms with its diamagnetic or paramagnetic behavior should be given by knowing their ground-state electron configurations.
Concept Introduction:
An orbital is an area of space in which electrons are orderly filled. The maximum capacity in any type of orbital is two electrons. An atomic orbital is defined as the region of space in which the probability of finding the electrons is highest. It is subdivided into four orbitals such as
There are three basic principles in which orbitals are filled by the electrons.
- 1. Aufbau principle: In German, the word 'aufbau' means 'building up'. The electrons are arranged in various orbitals in the order of increasing energies.
- 2. Pauli exclusion principle: An electron does not have all the four quantum numbers.
- 3. Hund’s rule: Each orbital is singly engaged with one electron having the maximum same spin capacity after that only pairing occurs.
The electron configuration is the allocation of electrons of an atom in atomic orbitals. Electronic configuration of a particular atom is written by following the three basic principles. If all the atomic orbitals are filled by electrons, then the atom is diamagnetic in nature. Diamagnetic atoms are repelled by the magnetic field. If one or more unpaired electrons are present in an atom, then that atom is paramagnetic in nature. Paramagnetic atoms are attracted to the magnetic field.
To find: Count the number of unpaired electrons in
Answer to Problem 3.104QP
The number of unpaired electron in
Explanation of Solution
The noble gas core for
Put all the electrons in the atomic orbitals by following Aufbau principle, Pauli exclusion principle and Hund’s rule.
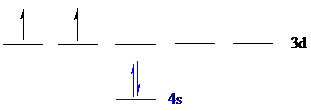
The four electrons of
The unpaired electrons are present in
Want to see more full solutions like this?
Chapter 3 Solutions
Chemistry: Atoms First - With Access
- How many unpaired electrons are there in an atom of (a) phosphorus? (b) potassium? (c) plutonium (Pu)?arrow_forwardGive the possible values of a. the principal quantum number, b. the angular momentum quantum number, c. the magnetic quantum number, and d. the spin quantum number.arrow_forwardFor the following pairs of orbitals, indicate which is higher in energy in a many-electron atom. (a) 3s or 2P (b) 4s or 4d (c) 4f or 6s (d) 1s or 2sarrow_forward
- How many electrons in an atom can have the following quantum designation? (a) 1s (b) 4d, m l =0(c) n=5,l=2arrow_forwardInvestigating Energy Levels Consider the hypothetical atom X that has one electron like the H atom but has different energy levels. The energies of an electron in an X atom are described by the equation E=RHn3 where RH is the same as for hydrogen (2.179 1018 J). Answer the following questions, without calculating energy values. a How would the ground-state energy levels of X and H compare? b Would the energy of an electron in the n = 2 level of H be higher or lower than that of an electron in the n = 2 level of X? Explain your answer. c How do the spacings of the energy levels of X and H compare? d Which would involve the emission of a higher frequency of light, the transition of an electron in an H atom from the n = 5 to the n = 3 level or a similar transition in an X atom? e Which atom, X or H, would require more energy to completely remove its electron? f A photon corresponding to a particular frequency of blue light produces a transition from the n = 2 to the n = 5 level of a hydrogen atom. Could this photon produce the same transition (n = 12 to n = 5) in an atom of X? Explain.arrow_forwardWithout looking at data in the text, sketch a qualitative graph of the third ionization energy versus atomic number for the elements Na through Ar, and explain your graph.arrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning  Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning





