
(a)
Interpretation:
An explanation regarding the arrangement of the compounds in order of their decreasing
Concept introduction:
The
Answer to Problem 3.47AP
The arrangement of the order of decreasing
Explanation of Solution
The given compounds are shown below.
Figure 1
Compounds shown in Figure 1 are alcohols that mean acidic proton is bonded to oxygen.
Compound B contains two chlorine atoms which are electron-withdrawing group. Due to
Compound C contains only one chlorine atom which is an electron-withdrawing group Due to
Compound A contains one methyl group which is an electron-donating group. Due to
Therefore, the order of acidity of the compounds is shown below.
Figure 2
The
Therefore, the order of decreasing
Figure 3
The order of decreasing
The arrangement of the order of decreasing
(b)
Interpretation:
An explanation regarding the arrangement of the compounds in order of their decreasing
Concept introduction:
The
Answer to Problem 3.47AP
The arrangement of the order of decreasing
Explanation of Solution
The given compounds are shown below.
Figure 4
According to the elemental effect, the acidity increases as the
Compound A contains only chlorine atom which is an electron-withdrawing group. Due to
Compound C contains
Therefore, the order of acidity of the compounds is shown below.
Figure 5
The
Therefore, the order of decreasing
Figure 6
The order of decreasing
The arrangement of the order of decreasing
(c)
Interpretation:
An explanation regarding the arrangement of the compounds in order of their decreasing
Concept introduction:
The
Answer to Problem 3.47AP
The arrangement of the order of decreasing
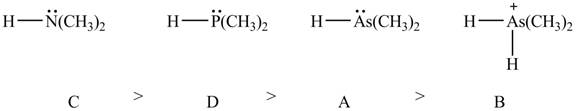
Explanation of Solution
The given compounds are shown below.
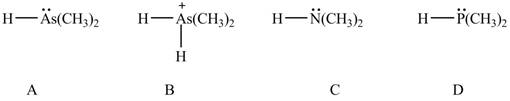
Figure 7
According to the elemental effect, the acidity increases as the atomic number attached to the acidic hydrogen also increases. The element
The atomic number of arsenic is higher than nitrogen and phosphorus. The
The atomic number of phosphorus is higher than nitrogen. The
According to the charge effect, the acidity increases with the presence of positive charge on atom attached to acidic hydrogen. Compound B contains a positive charge. Therefore, compound B is more acidic than compound A.
Therefore, the order of acidity of the compounds is shown below.
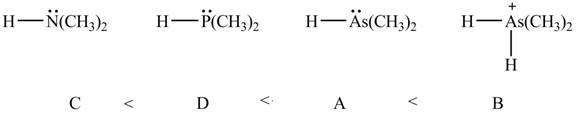
Figure 8
The
Therefore, the order of decreasing
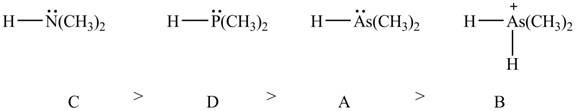
Figure 9
The order of decreasing
The arrangement of the order of decreasing
(d)
Interpretation:
An explanation regarding the arrangement of the compounds in order of their decreasing
Concept introduction:
The
Answer to Problem 3.47AP
The arrangement of the order of decreasing
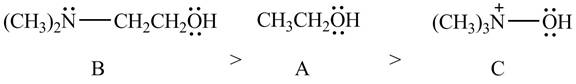
Explanation of Solution
The given compounds are shown below.
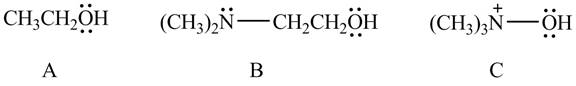
Figure 10
According to the charge effect, the acidity increases with the presence of positive charge on atom attached to acidic hydrogen. Compound C contains a positive charge. Therefore, compound C is more acidic than compound A and B.
Compound B contains only
Therefore, the order of acidity of the compounds is shown below.
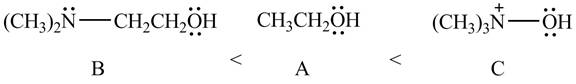
Figure 11
The
Therefore, the order of decreasing
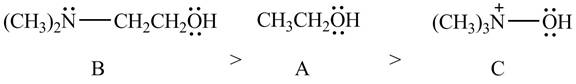
Figure 12
The order of decreasing
The arrangement of the order of decreasing
Want to see more full solutions like this?
Chapter 3 Solutions
Loose-leaf Version For Organic Chemistry
- Complete each acid-base reaction and predict whether the position of equilibrium lies toward the left or toward the right. (a) CH3CCH+CH3CH2ONa+CH3CH3OH (b) CH3CCCH2CH2OH+Na+NH2NH3(l)arrow_forwardThe following pKa values have been measured. Explain why a hydroxyl group in the para position decreases the acidity while a hydroxyl group in the meta position increases the acidity.arrow_forwardArrange the following organic molecules in order of increasing acidity, starting with the least acidic and explain your answer CH3CH3, HC≡CH and CH2=CH2arrow_forward
- dentify the conjugate base in the reaction of propanoic acid CH3CH3COOH reacting with methyl amine, CH3NH2. a. CH3NH2 b. CH3CH3COOH c. CH3NH3+ d. OH-1 e. CH3CH3COO-1arrow_forward(i) Arrange the following compounds in an increasing order of basic strength :C6H5NH2, C6H5N(CH3)2, (C2H5)2NH and CH3NH2(ii) Arrange the following compounds in a decreasing order of pKb values :C2H5NH2, C6H5NHCH3, (C2H5)2NH and C6H5NHarrow_forwardRank each of the following sets of nitrogen bases in terms of basicity and explain your answerarrow_forward
- Part 1. Choose the stronger acid in each pair of compounds. Part 2. Arrange the following compounds in order of increasing basicity. Part 3. Determine if the oxide is acidic or basearrow_forwardPredict the stronger acid between the pair of acids and explain your answer. a.I3CCH2CH2COOH and CH3CH2CCL2COOH.arrow_forwardArrange the following molecules in increasing order of acidity. Base it only on their structural differences and explain how it is so. 1. HF, CH3CH2CH2OH, CH3CH2COOH 2. Ethyl amine, Ethanol, Propanearrow_forward
- If the G for a reaction is 4.5 kcal/mol at 298 K, what is the Keq for this reaction? What is the change in entropy of this reaction if H = 3.2 kcal/mol?arrow_forwardThe acids are: isopropanol, ethyne, and phenol. Which acid will NOT be deprotanated by NaOH and which acid will deprotonate in the presence of NaHCO3. After an acid base reaction, which compound above has the weakest conjugate base?arrow_forwardRank the following compounds in order of increasing acidity (1 = least acidic, 3 = most acidic) and in the space provided use resonance (of the conjugate base) to explain why the compound you have labelled “3” is the most acidic.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

