
Concept explainers
How many protons, neutrons, and electrons are present in atoms with the following characteristics?
- a.
atomic number = 7, mass number = 15 - b. atomic number = 20, mass number = 40
- c. Z = 11, A = 23
- d. Z = 35, A = 79
(a)
Interpretation:
The number of protons, neutrons and electrons present in the atom that has atomic number of 7 and mass number of 15 has to be given.
Concept Introduction:
Atomic number for each and every element is a unique one. This is the total number of protons that is present in an atom. As the atom is electrically neutral, it can also be said that the total number of electrons is the atomic number. Atomic number is represented by the symbol Z.
Mass number is the sum of the number of protons and neutrons inside the nucleus of an atom. This gives the number of subatomic particle present inside the nucleus. Mass number is represented by the symbol A.
From atomic number and mass number, the number of each sub atomic particle can be found.
Complete chemical symbol notation can be given as.
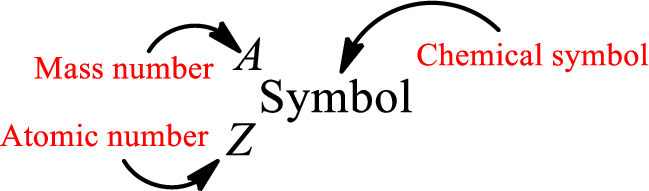
An element is a pure substance that cannot be broken by ordinary chemical reactions into simpler substances. All the atoms in an element will have the same atomic number. The electrons only take part in the chemical reaction while the nucleus does not. Hence, the atomic number (number or protons) does not change and it characterizes an atom.
Explanation of Solution
Given atom is said to have atomic number as 7 and mass number as 15. The number of protons, electrons and neutrons can be found as shown below,
Atomic number is given as 7 and mass number is given as 15.
Therefore, the number of protons is 7, number of electrons is 7, and number of neutrons is 8.
The number of protons, electrons and neutrons present in the atom is given.
(b)
Interpretation:
The number of protons, neutrons and electrons present in the atom that has atomic number of 20 and mass number of 40 has to be given.
Concept Introduction:
Atomic number for each and every element is a unique one. This is the total number of protons that is present in an atom. As the atom is electrically neutral, it can also be said that the total number of electrons is the atomic number. Atomic number is represented by the symbol Z.
Mass number is the sum of the number of protons and neutrons inside the nucleus of an atom. This gives the number of subatomic particle present inside the nucleus. Mass number is represented by the symbol A.
From atomic number and mass number, the number of each sub atomic particle can be found.
Complete chemical symbol notation can be given as.
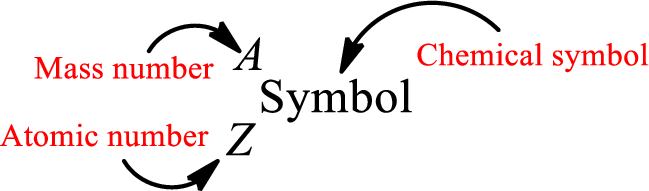
An element is a pure substance that cannot be broken by ordinary chemical reactions into simpler substances. All the atoms in an element will have the same atomic number. The electrons only take part in the chemical reaction while the nucleus does not. Hence, the atomic number (number or protons) does not change and it characterizes an atom.
Explanation of Solution
Given atom is said to have atomic number as 20 and mass number as 40. The number of protons, electrons and neutrons can be found as shown below,
Atomic number is given as 20 and mass number is given as 40.
Therefore, the number of protons is 20, number of electrons is 20, and number of neutrons is 20.
The number of protons, electrons and neutrons present in the atom is given.
(c)
Interpretation:
The number of protons, neutrons and electrons present in the atom which has Z as 11 and A as 23 has to be given.
Concept Introduction:
Atomic number for each and every element is a unique one. This is the total number of protons that is present in an atom. As the atom is electrically neutral, it can also be said that the total number of electrons is the atomic number. Atomic number is represented by the symbol Z.
Mass number is the sum of the number of protons and neutrons inside the nucleus of an atom. This gives the number of subatomic particle present inside the nucleus. Mass number is represented by the symbol A.
From atomic number and mass number, the number of each sub atomic particle can be found.
Complete chemical symbol notation can be given as.
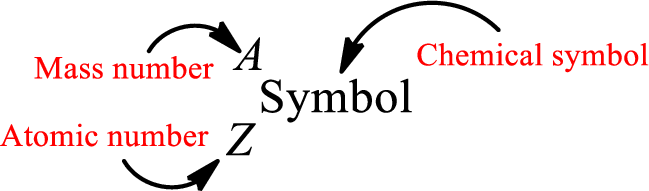
An element is a pure substance that cannot be broken by ordinary chemical reactions into simpler substances. All the atoms in an element will have the same atomic number. The electrons only take part in the chemical reaction while the nucleus does not. Hence, the atomic number (number or protons) does not change and it characterizes an atom.
Explanation of Solution
Given atom is said to have Z as 11 and A as 23. The number of protons, electrons and neutrons can be found as shown below,
Atomic number is given as 11 and mass number is given as 23.
Therefore, the number of protons is 11, number of electrons is 11, and number of neutrons is 12.
The number of protons, electrons and neutrons present in the atom is given.
(d)
Interpretation:
The number of protons, neutrons and electrons present in the atom which has Z as 35 and A as 79 has to be given.
Concept Introduction:
Atomic number for each and every element is a unique one. This is the total number of protons that is present in an atom. As the atom is electrically neutral, it can also be said that the total number of electrons is the atomic number. Atomic number is represented by the symbol Z.
Mass number is the sum of the number of protons and neutrons inside the nucleus of an atom. This gives the number of subatomic particle present inside the nucleus. Mass number is represented by the symbol A.
From atomic number and mass number, the number of each sub atomic particle can be found.
Complete chemical symbol notation can be given as.
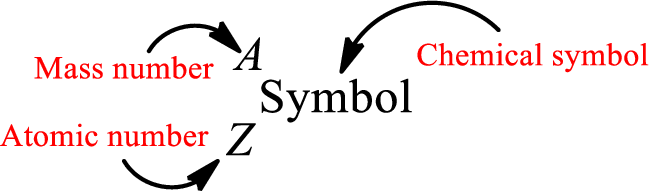
An element is a pure substance that cannot be broken by ordinary chemical reactions into simpler substances. All the atoms in an element will have the same atomic number. The electrons only take part in the chemical reaction while the nucleus does not. Hence, the atomic number (number or protons) does not change and it characterizes an atom.
Explanation of Solution
Given atom is said to have Z as 35 and A as 79. The number of protons, electrons and neutrons can be found as shown below,
Atomic number is given as 35 and mass number is given as 79.
Therefore, the number of protons is 35, number of electrons is 35, and number of neutrons is 34.
The number of protons, electrons and neutrons present in the atom is given.
Want to see more full solutions like this?
Chapter 3 Solutions
CHM 104/201 <C>
- The number of protons in an atom determines the identity of the atom. What do the number and arrangement of the electrons in an atom determine? What does the number of neutrons in an atom determine?arrow_forwardDo the proton and the neutron have exactly the same mass? How do the masses of the proton and the neutron compare to the mass of the electron? Which particles make the greatest contribution W the mass of an atom? Which particles make the greatest contribution to the chemical properties of an atom?arrow_forwardThe existence of isotopes violates one of the original ideas of Dalton’s atomic theory. Which one?arrow_forward
- Which particles in an atom are most responsible for the chemical properties of the atom? Where are these particles located in the atom?arrow_forwardHow many protons and neutrons are contained in the nucleus of each of the following atoms? For an atom of the element, how many electrons are present? a.2963Cu b.3580Br c.1224Mgarrow_forwardCarbon A carbon atom has a mass number of 12 and an atomic number of 6. How many neutrons does it have?arrow_forward
- Which of the following is(are) correct? a.40Ca2+ contains 20 protons and 18 electrons. b. Rutherford created the cathode-ray tube and was the founder of the charge-to-mass ratio of an electron. c. An electron is heavier than a proton. d. The nucleus contains protons, neutrons, and electrons.arrow_forwardExplain the operation of a cathode-ray tube. Describe the deflection of cathode rays by electrically charged plates placed within the cathode-ray tube. What does this imply about cathode rays?arrow_forwardAn atom that has lost three electrons will have a charge of .arrow_forward
- It is good practice to actively read the textbook and to try to verify claims that are made when you can. The following claim is made in your textbook: “. . . if the nucleus were the size of a grape, the electrons would be about 1 mile away on average.” Provide mathematical support for this statement.arrow_forwardHow many electrons, protons, and neutrons arecontained in each atom? a. 55132Cs c. 69163Tm b. 2759Cod. 3070Znarrow_forwardWhat is the mass number of an atom with 60 protons, 60 electrons, and 75 neutrons? a. 120 b. 135 c. 75 d. 195arrow_forward
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





