
3-23 Predict which ions are stable:
(a) 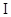
(b) 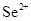
(c) 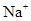
(d) 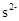
(e) 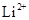
(f) 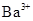
Interpretation:
Whether
Concept Introduction:
Octet rule: generally all atoms will lose, gain or share electrons to attain 8 valence electrons; the electronic configuration of the nearest noble element.
Duet rule: generally some atoms like hydrogen, lithium, beryllium will lose, gain or share electrons to attain 2 valence electrons; the electronic configuration of the nearest noble element like helium.
Answer to Problem 7P
Explanation of Solution
The atomic number or number of electrons of
When
The electronic configuration of the nearest noble element like helium, neon argon is the stable configuration hence the
Interpretation:
Whether
Concept Introduction:
Octet rule: generally all atoms will lose, gain or share electrons to attain 8 valence electrons; the electronic configuration of the nearest noble element.
Duet rule: generally some atoms like hydrogen, lithium, beryllium will lose, gain or share electrons to attain 2 valence electrons; the electronic configuration of the nearest noble element like helium.
Answer to Problem 7P
Explanation of Solution
The atomic number or number of electrons of
When
The electronic configuration of the nearest noble element like helium, neon argon is the stable configuration hence the
Interpretation:
Whether
Concept Introduction:
Octet rule: generally all atoms will lose, gain or share electrons to attain 8 valence electrons; the electronic configuration of the nearest noble element.
Duet rule: generally some atoms like hydrogen, lithium, beryllium will lose, gain or share electrons to attain 2 valence electrons; the electronic configuration of the nearest noble element like helium.
Answer to Problem 7P
Explanation of Solution
The atomic number or number of electrons of
When
The electronic configuration of the nearest noble element like helium, neon argon is the stable configuration hence the
Interpretation:
Whether
Concept Introduction:
Octet rule: generally all atoms will lose, gain or share electrons to attain 8 valence electrons; the electronic configuration of the nearest noble element.
Duet rule: generally some atoms like hydrogen, lithium, beryllium will lose, gain or share electrons to attain 2 valence electrons; the electronic configuration of the nearest noble element like helium.
Answer to Problem 7P
Explanation of Solution
The atomic number or number of electrons of
When
The electronic configuration of the nearest noble element like helium, neon argon is the stable configuration hence the
Interpretation:
Whether
Concept Introduction:
Octet rule: generally all atoms will lose, gain or share electrons to attain 8 valence electrons; the electronic configuration of the nearest noble element.
Duet rule: generally some atoms like hydrogen, lithium, beryllium will lose, gain or share electrons to attain 2 valence electrons; the electronic configuration of the nearest noble element like helium.
Answer to Problem 7P
Explanation of Solution
The atomic number or number of electrons of
When
The electronic configuration of the nearest noble element like helium, neon argon is the stable configuration hence the
Interpretation:
Whether
Concept Introduction:
Octet rule: generally all atoms will lose, gain or share electrons to attain 8 valence electrons; the electronic configuration of the nearest noble element.
Duet rule: generally some atoms like hydrogen, lithium, beryllium will lose, gain or share electrons to attain 2 valence electrons; the electronic configuration of the nearest noble element like helium.
Answer to Problem 7P
Explanation of Solution
The atomic number or number of electrons of
When
The electronic configuration of the nearest noble element like helium, neon argon is the stable configuration hence the
Want to see more full solutions like this?
Chapter 3 Solutions
Introduction to General, Organic and Biochemistry
- 3-109 Until several years ago, the two chlorofluorocarbons (CFCs) most widely used as heat transfer media in refrigeration systems were Freon-li (trichloro fluoromethane, CC13F) and Freon-12 (dichiorodi fluoromethane, CCl2F2). Draw a three-dimensional representation of each molecule and indicate the Direction of it.s polarity.arrow_forward3-67 Why does nitrogen have three bonds and one unshared pair of electrons in covalent compounds?arrow_forward3-48 Potassium chloride and potassium bicarbonate are used as potassium dietary supplements. Write the formula of each compound.arrow_forward
- 3-24 Predict which ions are stable: (a) Br2- (b) C4- (c) Ca+ (d) Ar+ (e) Na+ (f) Cs+arrow_forward3-87 Consider the molecule boron trffluoride, BF3. (a) Write a Lewis structure for BF3. (b) Predict the FBF bond angles using the VSEPR model. (c) Does BF3 have polar bonds? Is it a polar molecule?arrow_forward3-63 What is the difference between (a) a bromine atom, (b) a bromine molecule, and (c) a bromide ion? Draw the Lewis structure for each.arrow_forward
- 3-58 In Section 2-3B, we saw that there are seven diatomic elements. (a) Draw Lewis structures for each of these diatomic elements. (b) Which diatomic elements are gases at room temperature? Which are liquids? Which are solids?arrow_forward3-56 How many covalent bonds are normally formed by each element? (a)N ( b)F (c)C (d)Br (e)Oarrow_forward2-98 Explain how the ionization energy of atoms changes when proceeding down a group of the Periodic Table and explain why this change occurs.arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
