
Package: Loose Leaf for Organic Chemistry with Biological Topics with Connect Access Card
5th Edition
ISBN: 9781260170405
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 30, Problem 30.59P
Although chain branching in radical
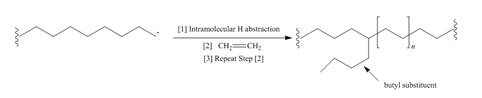
a. Draw a stepwise mechanism that illustrates which H must be intramolecularly abstracted to form butyl substituents.
b. Suggest a reason why the abstraction of this H is more facile than the abstraction of other H’s.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Although chain branching in radical polymerizations can occur by intermolecular H abstraction as shown in Mechanism 28.2, chain branching can also occur by intramolecular H abstraction to form branched polyethylene that contains butyl groups as branches.a. Draw a stepwise mechanism that illustrates which H must be intramolecularly abstracted to form butyl substituents. b. Suggest a reason why the abstraction of this H is more facile than the abstraction of other H's.
(a) Which monomer is most likely to undergo anionic polymerization? Justify your choice.
( b)Which one ismost likely to undergo cationic polymerization? Justify your choice.
Propylene forms only oligomer under normal free radical conditions, yet the monomer forms high-molecular-weight polymer if it is polymerized while trapped in the channels of a crystalline host substance such as urea or thiourea. Explain [G. Di Silvestro, P. Sozzani, and M. Farina, Polym. Preprints, 27(1), 92, 1986].
Chapter 30 Solutions
Package: Loose Leaf for Organic Chemistry with Biological Topics with Connect Access Card
Ch. 30 - Prob. 30.1PCh. 30 - Prob. 30.2PCh. 30 - Prob. 30.3PCh. 30 - Draw the mechanism for the radical polymerization...Ch. 30 - Prob. 30.5PCh. 30 - Prob. 30.6PCh. 30 - Prob. 30.7PCh. 30 - Prob. 30.8PCh. 30 - Prob. 30.9PCh. 30 - Prob. 30.10P
Ch. 30 - Prob. 30.11PCh. 30 - Problem 30.12
What polymer is formed by anionic...Ch. 30 - Prob. 30.13PCh. 30 - Prob. 30.14PCh. 30 - Problem 30.15
What polyamide is formed from each...Ch. 30 - Prob. 30.16PCh. 30 - Prob. 30.17PCh. 30 - Prob. 30.18PCh. 30 - Prob. 30.19PCh. 30 - Prob. 30.20PCh. 30 - Prob. 30.21PCh. 30 - Prob. 30.22PCh. 30 - Prob. 30.23PCh. 30 - Prob. 30.24PCh. 30 - Prob. 30.25PCh. 30 - 30.26 Draw the structure of the polymer formed by...Ch. 30 - Prob. 30.27PCh. 30 - Prob. 30.28PCh. 30 - Prob. 30.29PCh. 30 - 30.30 Draw each polymer in Problem 30.29 using the...Ch. 30 - Prob. 30.31PCh. 30 - Prob. 30.32PCh. 30 - Prob. 30.33PCh. 30 - Prob. 30.34PCh. 30 - Prob. 30.35PCh. 30 - Prob. 30.36PCh. 30 - Prob. 30.37PCh. 30 - Prob. 30.38PCh. 30 - 30.39 Draw a stepwise mechanism for the...Ch. 30 - 30.40 Cationic polymerization of 3-phenylpropene ...Ch. 30 - Prob. 30.41PCh. 30 - Prob. 30.42PCh. 30 - 30.43 Although styrene undergoes both cationic and...Ch. 30 - 30.44 Rank the following compounds in order of...Ch. 30 - Prob. 30.45PCh. 30 - Prob. 30.46PCh. 30 - 30.47 Draw a stepwise mechanism for the following...Ch. 30 - 30.48 Draw a stepwise mechanism for the reaction...Ch. 30 - 30.49 Draw the products of each reaction.
a. e....Ch. 30 - Prob. 30.50PCh. 30 - Prob. 30.51PCh. 30 - 30.52 (a) Explain why poly (vinyl alcohol) cannot...Ch. 30 - 30.53 Devise a synthesis of terephthalic acid and...Ch. 30 - Prob. 30.54PCh. 30 - Prob. 30.55PCh. 30 - 30.56 Compound A is a novel poly (ester amide)...Ch. 30 - 30.57 Researchers at Rutgers University have...Ch. 30 - 30.58 Melmac, a thermosetting polymer formed from...Ch. 30 - 30.59 Although chain branching in radical...Ch. 30 - Prob. 30.60P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- a) Please draw the retrosynthesis of this polymer below.b) Draw the structure of another polymer that can react with this polymer to generate soft material.c) Draw the structure of a cross-linker could be used for the reaction in question b). .arrow_forwardDraw Anionic polymerization mechanism for the next reaction, poly (methyl methacrylate) (PMMA) Prepared with anionic catalyst (Florinetium or Greniard reagents *The picture is for reference only*arrow_forwardDraw the curved arrow mechanism for the first step in the formation of one repeat unit of this polymer.arrow_forward
- (a) Show how poly(propylene oxide) can be synthesized via anionic ring-opening polymerization. (b) Draw the mechanism for the initiation and first two propagation steps.arrow_forwardDraw a stepwise mechanism for the following polymerization reaction.arrow_forwardPropylene does not undergo free radical polymerization readily because there are two competing steps after initiation: propagation and hydrogen atom abstraction.(a) Using a generic radical R• as a reactant with propylene, draw the mechanism and products for the two competing steps.(b) Which step produces the more stable product?(c) How do your results explain propylene’s poor reactivity in free radical polymerization?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
CBSE Class 12 Chemistry || Polymers || Full Chapter || By Shiksha House; Author: Best for NEET;https://www.youtube.com/watch?v=OxdJlS0xZ0Y;License: Standard YouTube License, CC-BY