
Organic Chemistry - With Access (Custom)
4th Edition
ISBN: 9781259147760
Author: OHIO UNIV.
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 31, Problem 31.53P
(a) Explain why poly (vinyl alcohol) cannot be prepared by the radical 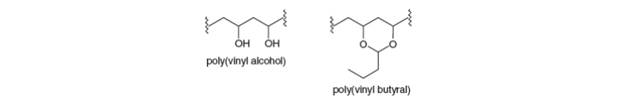
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Poly(vinyl alcohol) is a polymer used to make fibers and adhesives. It is synthesized by hydrolysis or alcoholysis of the polymer obtained from polymerization of vinyl acetate as shown below. a. Why is poly(vinyl alcohol) not prepared by polymerizing vinyl alcohol? b. Is poly(vinyl acetate) a polyester?
Which reaction—dehydration synthesis or hydrolysis—converts a polymer to its monomers? Which one convertsmonomers to a polymer? Explain your answer
Draw the starting structure that would lead to this polymer under radical cond
Chapter 31 Solutions
Organic Chemistry - With Access (Custom)
Ch. 31 - Prob. 31.1PCh. 31 - Prob. 31.2PCh. 31 - Prob. 31.3PCh. 31 - Draw the mechanism for the radical polymerization...Ch. 31 - Prob. 31.5PCh. 31 - Prob. 31.6PCh. 31 - Prob. 31.7PCh. 31 - Prob. 31.8PCh. 31 - Prob. 31.9PCh. 31 - Prob. 31.10P
Ch. 31 - Prob. 31.11PCh. 31 - Problem 30.12
What polymer is formed by anionic...Ch. 31 - Prob. 31.13PCh. 31 - Prob. 31.14PCh. 31 - Problem 30.15
What polyamide is formed from each...Ch. 31 - Prob. 31.16PCh. 31 - Prob. 31.17PCh. 31 - Prob. 31.18PCh. 31 - Prob. 31.19PCh. 31 - Prob. 31.20PCh. 31 - Prob. 31.21PCh. 31 - Prob. 31.22PCh. 31 - Prob. 31.23PCh. 31 - Prob. 31.24PCh. 31 - Prob. 31.25PCh. 31 - Prob. 31.26PCh. 31 - 30.26 Draw the structure of the polymer formed by...Ch. 31 - Prob. 31.28PCh. 31 - Prob. 31.29PCh. 31 - Prob. 31.30PCh. 31 - Prob. 31.31PCh. 31 - Prob. 31.32PCh. 31 - Prob. 31.33PCh. 31 - Prob. 31.34PCh. 31 - Prob. 31.35PCh. 31 - Prob. 31.36PCh. 31 - Prob. 31.37PCh. 31 - Prob. 31.38PCh. 31 - Prob. 31.39PCh. 31 - 30.39 Draw a stepwise mechanism for the...Ch. 31 - 30.40 Cationic polymerization of 3-phenylpropene ...Ch. 31 - Prob. 31.42PCh. 31 - Prob. 31.43PCh. 31 - 30.43 Although styrene undergoes both cationic and...Ch. 31 - 30.44 Rank the following compounds in order of...Ch. 31 - Prob. 31.46PCh. 31 - Prob. 31.47PCh. 31 - 30.47 Draw a stepwise mechanism for the following...Ch. 31 - 30.48 Draw a stepwise mechanism for the reaction...Ch. 31 - Prob. 31.50PCh. 31 - Prob. 31.51PCh. 31 - Prob. 31.52PCh. 31 - 30.52 (a) Explain why poly (vinyl alcohol) cannot...Ch. 31 - Prob. 31.54PCh. 31 - 30.53 Devise a synthesis of terephthalic acid and...Ch. 31 - Prob. 31.56PCh. 31 - Prob. 31.57PCh. 31 - 30.56 Compound A is a novel poly (ester amide)...Ch. 31 - Researchers at Rutgers University have developed...Ch. 31 - 30.58 Melmac, a thermosetting polymer formed from...Ch. 31 - 30.59 Although chain branching in radical...Ch. 31 - Prob. 31.62P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- List the following group of monomers in order of decreasing ability to undergo cationic polymerization.arrow_forwardExplain the Mechanism - Forming Branched Polyethylene During RadicalPolymerization ?arrow_forwardThe polymerization of CH2 = CHCH = CH2 under radical conditions affords products A and B. Draw a mechanism that accounts for their formation.arrow_forward
- Cationic polymerization of 3-phenylpropene (CH2=CHCH2Ph) affords Aas the major product rather than B. Draw a stepwise mechanism toaccount for this observation.arrow_forwardChain branching occurs in cationic polymerization much as it does in free-radical polymerization. Propose a mechanism to show how branching occurs in the cationic polymerization of styrene. Suggest why isobutylene might be a better monomer for cationic polymerization than styrene.arrow_forwardDraw a structural formula of the polymer resulting from base-catalyzed polymerization of each compound. Would you expect the polymers to be optically active? (S)-(+)-lactide is the dilactone formed from two molecules of (S)-(+)-lactic acid.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT

CBSE Class 12 Chemistry || Polymers || Full Chapter || By Shiksha House; Author: Best for NEET;https://www.youtube.com/watch?v=OxdJlS0xZ0Y;License: Standard YouTube License, CC-BY