
Organic Chemistry (6th Edition)
6th Edition
ISBN: 9781260119107
Author: Janice Gorzynski Smith
Publisher: McGraw Hill Education
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 3.2, Problem 2P
(a) Classify the carbon atoms in each compound as
[1] 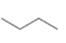 [2]
[2] [3]
[3]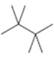 [4]
[4]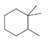
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Explain the following statement. Although HC ≡ C– is more stable than CH2 = CH–, HC ≡ C+ is less stable than CH2 = CH+.
Answer each question using the ball-and-stick model of compound A.
Draw a stereoisomer for A and give its IUPAC name.Draw a constitutional isomer that contains an OH group and give itsIUPAC name.
Classify each pair of compounds as constitutional isomers or identical molecules.
Chapter 3 Solutions
Organic Chemistry (6th Edition)
Ch. 3.1 - Prob. 1PCh. 3.2 - (a) Classify the carbon atoms in each compound as...Ch. 3.2 - Problem 3.3 Classify a carbon atom by the number...Ch. 3.2 - Classify each alkyl halide and alcohol as , or...Ch. 3.2 - Prob. 5PCh. 3.2 - Prob. 6PCh. 3.2 - Draw the structure of a compound of molecular...Ch. 3.2 - Prob. 8PCh. 3.2 - Prob. 9PCh. 3.2 - Draw the structure of a compound fitting each...
Ch. 3.4 - Predict which compound in each pair has the higher...Ch. 3.4 - Prob. 17PCh. 3.4 - a Label the hydrophobic and hydrophilic portions...Ch. 3.5 - Prob. 21PCh. 3 - 3.29
Identify the functional groups in the...Ch. 3 - Prob. 32PCh. 3 - 3.31 For each alkane: (a) classify each carbon...Ch. 3 - 3.32 Identify the functional groups in each...Ch. 3 - 3.33 Identify each functional group located in the...Ch. 3 - 3.34 (a)Identify the functional groups in...Ch. 3 - Draw seven constitutional isomers with molecular...Ch. 3 - Prob. 38PCh. 3 - Prob. 39PCh. 3 - Prob. 40PCh. 3 - Intramolecular force of attraction are often...Ch. 3 - 3.40 (a) Draw four compounds with molecular...Ch. 3 - 3.41 Rank the compounds in each group in order of...Ch. 3 - Explain why CH3CH2NHCH3 has higher boiling point...Ch. 3 - Prob. 45PCh. 3 - 3.44 Rank the following compounds in order of...Ch. 3 - Prob. 47PCh. 3 - 3.50 Predict the solubility of each of the...Ch. 3 - Prob. 52PCh. 3 - Prob. 53PCh. 3 - 3.53 THC is the active component in marijuana, and...Ch. 3 - Prob. 55PCh. 3 - Prob. 56PCh. 3 - 3.60 Quinapril (trade name Accupril) is a drug...Ch. 3 - 3.61 Answer each question about oxycodone, a...Ch. 3 - Prob. 65PCh. 3 - Prob. 66PCh. 3 - 3.64 Explain why A is less water soluble than B,...Ch. 3 - 3.65 Recall from section 1.10B that there is...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Label each pair of compounds as constitutional isomers, stereoisomers, or not isomers of each other.arrow_forwardClassify each pair of compounds as constitutional isomers, stereoisomers, identical molecules, or not isomers of each other.arrow_forwardWhich compound in each pair has the higher boiling point? Explainarrow_forward
- Classify each attached pair of compounds as constitutional isomers or identical molecules.arrow_forward"A cis alkene is more polar than a trans alkene, giving it a slightlyhigher boiling point and making it more soluble in polar solvents" Explain this statement ?arrow_forwardClassify each pair of compounds as constitutional isomers, stereoisomers, identical molecules, or not isomers of each attached otherarrow_forward
- (a) Draw a skeletal structure of the anabolic steroid methenolone from the following description. Methenolone contains the tetracyclic steroid skeleton with a carbonyl group at C3, a hydroxyl at C17, a double bond between C1 and C2, and methyl groups bonded to C1, C10, and C13. (b) Add wedges and dashed wedges for all stereogenic centers with thefollowing information: the configuration at C10 is R, the configuration at C13 is S, the configuration at C17 is S, and all substituents at ring fusions are trans to each other. (c) Draw the structure of Primobolan, the product formed when methenolone is treated with CH3(CH2)5COCl and pyridine. Primobolan is an anabolic steroid that can be taken orally or by injection and has been used illegally by well-known Major League Baseball players.arrow_forwardAnswer the following question about compounds A–D (See in attachment) How are the compounds in each pair related? Choose fromconstitutional isomers, stereoisomers, or identical molecules: A and B; A and C; B and D.arrow_forwardGive the IUPAC name for each sulde.arrow_forward
- Draw four constitutional isomers having molecular formula C 6H 12 that contain a four-membered ring. Give the IUPAC name for each isomer.arrow_forwarda) Identify three (3) chiral centers in compound Cb) Explain the solubility of polar organic compound in waterarrow_forwardDraw three constitutional isomers having molecular formula C 7H 14 that contain a fi ve-membered ring and two CH 3 groups bonded to the ring. Give the IUPAC name for each isomerarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY