
(a)
Interpretation:
The product, balanced equation, net ionic equation and the type of the given reaction should be identified.
Concept introduction:
Precipitation reaction: The formation of the product is insoluble when the ions combine in the solution is called precipitation reaction.
Acid - base reaction: Formation of the salt from the cation from the base and anion from the acid and formation of water is also the product.
Gas forming reaction: The reaction of acid and metal carbonates which produce carbonic acid. The carbonic acid decomposes which gives water and carbon dioxide.
Oxidation - reduction reaction: The electrons are transferred to one to other is called oxidation reduction reaction.
Most of the ionic compounds are soluble in water, very few of the ionic compounds are sparingly soluble, and some of the ionic compounds are insoluble in water. When it is soluble in water ions gets separated in the solution.
Soluble compounds in water
Almost all the salts of
Almost all the salts of
Salts of F- are soluble. But some of the fluoride salt of
Salts of
Insoluble compounds in water:
Most of the salts of
Most of the metal hydroxides and oxides are insoluble in water bit some of the alkali metal hydroxides,
(a)
Answer to Problem 3.10CYU
The product of the reaction, balancing of the reaction and the type of the reaction is shown below, the given reaction is gas forming reaction and the balanced equation is shown below
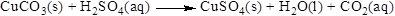
Net ionic equation of the given reaction shown below (a)
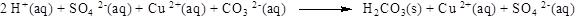
Explanation of Solution
The product of the reaction, balancing of the reaction and the type of the reaction is shown below, the given reaction is gas forming reaction and the balanced equation is shown below
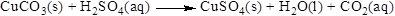
The given compound is copper carbonate and sulfuric acid which is soluble in water. In this reaction copper carbonate reaction with sulfuric acid to give copper sulfate and carbon dioxide and water.
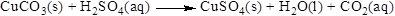
Balance the equation,
The reaction is already balanced. Therefore the balanced equation is given below.
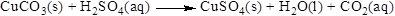
The net ionic equation is given below,
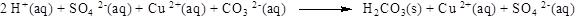
(b)
Interpretation:
The product, balanced equation, net ionic equation and the type of the given reaction should be identified.
Concept introduction:
Precipitation reaction: The formation of the product is insoluble when the ions combine in the solution is called precipitation reaction.
Acid - base reaction: Formation of the salt from the cation from the base and anion from the acid and formation of water is also the product.
Gas forming reaction: The reaction of acid and metal carbonates which produce carbonic acid. The carbonic acid decomposes which gives water and carbon dioxide.
Oxidation - reduction reaction: The electrons are transferred to one to other is called oxidation reduction reaction.
Most of the ionic compounds are soluble in water, very few of the ionic compounds are sparingly soluble, and some of the ionic compounds are insoluble in water. When it is soluble in water ions gets separated in the solution.
Soluble compounds in water
Almost all the salts of
Almost all the salts of
Salts of F- are soluble. But some of the fluoride salt of
Salts of
Insoluble compounds in water:
Most of the salts of
Most of the metal hydroxides and oxides are insoluble in water bit some of the alkali metal hydroxides,
(b)
Answer to Problem 3.10CYU
The product of the reaction, balancing of the reaction and the type of the reaction is shown below, the given reaction is oxidation-reduction reaction and the balanced equation is shown below (b)
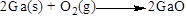
Net ionic equation of the given reaction shown below (b)
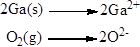
Explanation of Solution
The product of the reaction, balancing of the reaction and the type of the reaction is shown below, the given reaction is oxidation-reduction reaction and the balanced equation is shown below
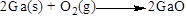
The given compound is gallium and oxygen. In this reaction gallium reaction with oxygen to give gallium oxide, sulfur dioxide. Here the oxidation state of gallium is zero in the reactant and
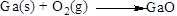
Balance the equation,
Balance the oxygen atom in the given equation, when balancing the equation, we should not alter the subscripts and we can change coefficients. There are two oxygen atoms in the left side and one oxygen atoms in the right side. Therefore two molecule of gallium oxide is added to right side of reaction. Therefore the balanced equation is given below.
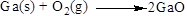
Balance the gallium atom in the given equation. There are two gallium atoms in the right side and one gallium atoms in the left side. Therefore two molecule of gallium is added to left side of reaction. Therefore the balanced equation is given below.
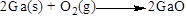
Net ionic equation of the given reaction shown below

(c)
Interpretation:
The product, balanced equation, net ionic equation and the type of the given reaction should be identified.
Concept introduction:
Precipitation reaction: The formation of the product is insoluble when the ions combine in the solution is called precipitation reaction.
Acid - base reaction: Formation of the salt from the cation from the base and anion from the acid and formation of water is also the product.
Gas forming reaction: The reaction of acid and metal carbonates which produce carbonic acid. The carbonic acid decomposes which gives water and carbon dioxide.
Oxidation - reduction reaction: The electrons are transferred to one to other is called oxidation reduction reaction.
Most of the ionic compounds are soluble in water, very few of the ionic compounds are sparingly soluble, and some of the ionic compounds are insoluble in water. When it is soluble in water ions gets separated in the solution.
Soluble compounds in water
Almost all the salts of
Almost all the salts of
Salts of F- are soluble. But some of the fluoride salt of
Salts of
Insoluble compounds in water:
Most of the salts of
Most of the metal hydroxides and oxides are insoluble in water bit some of the alkali metal hydroxides,
(c)
Answer to Problem 3.10CYU
The product of the reaction, balancing of the reaction and the type of the reaction is shown below, the given reaction is acid-base reaction and the balanced equation is shown below (c)
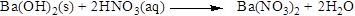
Net ionic equation of the given reaction shown below (c)
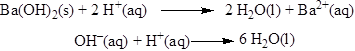
Explanation of Solution
The product of the reaction, balancing of the reaction and the type of the reaction is shown below, the given reaction is acid-base reaction and the balanced equation is shown below
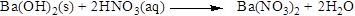
The given compound is barium hydroxide and nitric acid. In this reaction barium hydroxide reaction with nitric acid to give barium nitrate and formation of water is also the product.
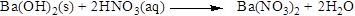
Balance the equation,
Balance the nitrogen atom in the given equation, when balancing the equation, we should not alter the subscripts and we can change coefficients. There are two nitrogen atoms in the right side and one nitrogen atoms in the left side. Therefore two molecule of nitric acid is added to left side of reaction. Therefore the balanced equation is given below.
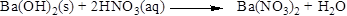
Balance the hydrogen atom in the given equation. There are two hydrogen atoms in the right side and four hydrogen atoms in the left side. Therefore two molecule of water is added to right side of reaction. Therefore the balanced equation is given below.
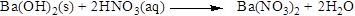
Net ionic equation of the given reaction shown below
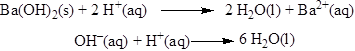
(d)
Interpretation:
The product, balanced equation, net ionic equation and the type of the given reaction should be identified.
Concept introduction:
Precipitation reaction: The formation of the product is insoluble when the ions combine in the solution is called precipitation reaction.
Acid - base reaction: Formation of the salt from the cation from the base and anion from the acid and formation of water is also the product.
Gas forming reaction: The reaction of acid and metal carbonates which produce carbonic acid. The carbonic acid decomposes which gives water and carbon dioxide.
Oxidation - reduction reaction: The electrons are transferred to one to other is called oxidation reduction reaction.
Most of the ionic compounds are soluble in water, very few of the ionic compounds are sparingly soluble, and some of the ionic compounds are insoluble in water. When it is soluble in water ions gets separated in the solution.
Soluble compounds in water
Almost all the salts of
Almost all the salts of
Salts of F- are soluble. But some of the fluoride salt of
Salts of
Insoluble compounds in water:
Most of the salts of
Most of the metal hydroxides and oxides are insoluble in water bit some of the alkali metal hydroxides,
(d)
Answer to Problem 3.10CYU
The product of the reaction, balancing of the reaction and the type of the reaction is shown below, the given reaction is precipitation reaction and the balanced equation is shown below.

Net ionic equation of the given reaction shown below (a)
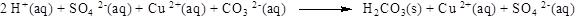
Explanation of Solution
The product of the reaction, balancing of the reaction and the type of the reaction is shown below, the given reaction is precipitation reaction and the balanced equation is shown below

The given compound is copper chloride and ammonium sulfide. In this reaction copper chloride reaction with ammonium sulfide to give copper sulfide and ammonium chloride.
the given reaction is precipitation reaction.

Balance the equation,
Balance the nitrogen atom in the given equation, when balancing the equation, we should not alter the subscripts and we can change coefficients. There are two nitrogen atoms in the left side and one nitrogen atoms in the right side. Therefore two molecule of ammoniu chloride is added to right side of reaction. Therefore the balanced equation is given below.

Want to see more full solutions like this?
Chapter 3 Solutions
CHEMISTRY+CHEM.REACT. (LOOSELEAF)
- Bone was dissolved in hydrochloric acid, giving 50.0 mL of solution containing calcium chloride, CaCL2. To precipitate the calcium ion from the resulting solution, an excess of potassium oxalate was added. The precipitate of calcium oxalate, CaC2O4, weighed 1.437 g. What was the molarity of CaCl2 in the solution?arrow_forwardMagnesium metal (a component of alloys used in aircraft and a reducing agent used in the production of uranium, titanium, and other active metals) is isolated from sea water by the following sequence of reactions: Mg2+(aq)+Ca(OH)2(aq)Mg(OH)2(s)+Ca2+(aq)Mg(OH)2(s)+2HCl(aq)MgCl2(s)+2H2O(l)MgCl2(l)electrolysisMg(s)+Cl2+Cl2(g) Sea water has a density of 1.026 g/cm3 and contains 1272 parts per million of magnesium a5 Mg2+(aq) by mass. What mass, in kilograms, of Ca(OH)2; is required to precipitate 99.9% of the magnesium in 1.00103 L of sea water?arrow_forwardGold metal will dissolve only in aqua regia, a mixture of concentrated hydrochloric acid and concentrated nitric acid in a 3:1 volume ratio. The products of the reaction between gold and the concentrated acids are AuCl4-(aq), NO(g), and H2O. The equation for this reaction where HNO3 and HCl are strong acids is Au(s)+4Cl(aq)+4H+(aq)+NO3(aq)AuCl4(aq)+NO(g)+2H2O(a) What stoichiometric ratio of hydrochloric acid to nitric acid should be used? (b) What volumes of 12 M HCl and 16 M are HNO3 required to furnish the Cl- and NO3- ions to react with 25.0 g of gold?arrow_forward
- Arsenic acid, H3AsO4, is a poisonous acid that has been used in the treatment of wood to prevent insect damage. Arsenic acid has three acidic protons. Say you take a 25.00-mL sample of arsenic acid and prepare it for titration with NaOH by adding 25.00 mL of water. The complete neutralization of this solution requires the addition of 53.07 mL of 0.6441 M NaOH solution. Write the balanced chemical reaction for the titration, and calculate the molarity of the arsenic acid sample.arrow_forward4-81 (Chemical Connections 4C) Balance the lithium iodine battery redox reaction described in this sec tion and identify the oxidizing and reducing agents present.arrow_forwardA soluble iodide was dissolved in water. Then an excess of silver nitrate, AgNO3, was added to precipitate all of the iodide ion as silver iodide, AgI. If 1.545 g of the soluble iodide gave 2.185 g of silver iodide, how many grams of iodine are in the sample of soluble iodide? What is the mass percentage of iodine, I, in the compound?arrow_forward
- A sample of limestone weighing 1.005 g is dissolved in 75.00 mL of 0.2500 M hydrochloric acid. The following reaction occurs: CaCO3(s)+2 H+(aq)Ca2+(aq)+CO2(g)+H2O It is found that 19.26 mL of 0.150 M NaOH is required to titrate the excess HCI left after reaction with the limestone. What is the mass percent of CaCO3 in the limestone?arrow_forwardA 1.345-g sample of a compound of barium and oxygen was dissolved in hydrochloric acid to give a solution of barium ion, which was then precipitated with an excess of potassium chromate to give 2.012 g of barium chromate, BaCrO4. What is the formula of the compound?arrow_forwardThe active ingredients of an antacid tablet contained only magnesium hydroxide and aluminum hydroxide. Complete neutralization of a sample of the active ingredients required 48.5 mL of 0.187 M hydrochloric acid. The chloride salts from this neutralization were obtained by evaporation of the filtrate from the titration; they weighed 0. 4200 g. What was the percentage by mass of magnesium hydroxide in the active ingredients of the antacid tablet?arrow_forward
- Aqueous solutions of ammonium sulfide and mercury(II) nitrate react and a precipitate forms. (a) Write the overall balanced chemical equation and indicate the state (aq) or (s) for each compound. (b) Name each product. (c) Write the complete ionic equation. (d) Write the net ionic equation.arrow_forwardChlorisondamine chloride (C14H20Cl6N2) is a drug used in the treatment of hypertension. A 1.28-g sample of a medication containing the drug was treated to destroy the organic material and to release all the chlorine as chloride ion. When the filtered solution containing chloride ion was treated with an excess of silver nitrate, 0.104 g silver chloride was recovered. Calculate the mass percent of chlorisondamine chloride in the medication, assuming the drug is the only source of chloride.arrow_forwardOn Easter Sunday, April 3, 1983, nitric acid spilled from a tank car near downtown Denver, Colorado. The spill was neutralized with sodium carbonate: 2HNO3(aq)+Na2CO3(aq)2NaNO3(aq)+H2O(l)+CO2(g) a. Calculate H for this reaction. Approximately 2.0 104 gal nitric acid was spilled. Assume that the acid was an aqueous solution containing 70.0% HNO3 by mass with a density of 1.42 glcm3. What mass of sodium carbonate was required for complete neutralization of the spill, and what quantity of heat was evolved? (Hf for NaNO3(aq) = 467 kJ/mol) b. According to The Denver Post for April 4, 1983, authorities feared that dangerous air pollution might occur during the neutralization. Considering the magnitude of H, what was their major concern?arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning





