
Organic Chemistry (Instructor's)
9th Edition
ISBN: 9781305082113
Author: McMurry
Publisher: Cengage
expand_more
expand_more
format_list_bulleted
Question
Chapter 3.SE, Problem 45AP
Interpretation Introduction
Interpretation:
The dipole moment of 1, 2-Dibromoethane is to be determined.
Concept introduction:
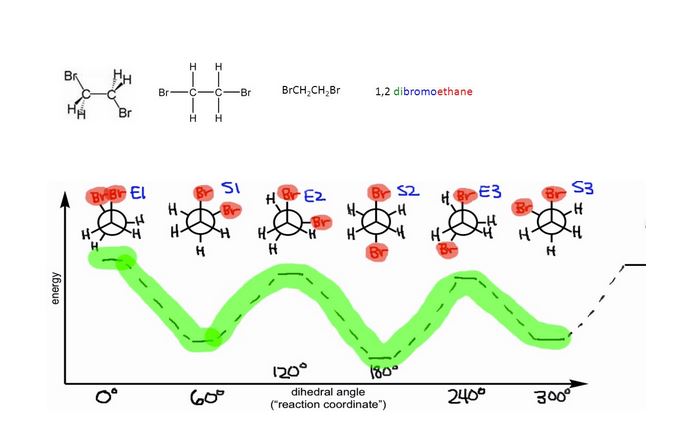
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
3-35
Each of the following descriptions applies to more than one alkane. In each case, draw and name two structures that match
the description.
(a) an isopropylheptane
(d) a trans-dihalocyclopentane
(b) a diethyldecane
(e) a (2,3-dimethylpentyl)cycloalkane
(c) a cis-diethylcyclohexane
(f) a bicyclononane
Below are isomers of tert-butylcyclohexanol in conformational structures. Two are cis-2-tert-
butylcyclohexanol and two are cis-3-tert-butylcyclohexanol. Indicate which is the more stable conformation
for each pair, and indicate which of all four conformations is the most stable.
A
OH
Ex
HO
B
D
OH
OH
Here is the chemical structure of 2-bromobutane:
Н Η Н Н
HHHH
C C- C C-H
H :Br Η Н
..
Decide whether each molecule in the table below is another molecule of 2-bromobutane, a molecule of an isomer of 2-bromobutane, or a molecule of an entirely
different compound.
molecule
CH₂
CH₂-CH₂-CH-Br
CH₂
CH₂ -CH₂
Br
HI
H
H
H Η
H_
H-C- C-C-
H-C-H
H
H
relationship to 2-bromobutane
(Choose one)
(Choose one)
Br:
a molecule of an isomer of 2-bromobutane ▼
Chapter 3 Solutions
Organic Chemistry (Instructor's)
Ch. 3.1 - Prob. 1PCh. 3.1 - Prob. 2PCh. 3.1 - Identify the functional groups in the following...Ch. 3.2 - Draw structures of the five isomers of C6H14.Ch. 3.2 - Propose structures that meet the following...Ch. 3.2 - Prob. 6PCh. 3.3 - Draw the eight 5-carbon alkyl groups (pentyl...Ch. 3.3 - Identify the carbon atoms in the following...Ch. 3.3 - Prob. 9PCh. 3.3 - Prob. 10P
Ch. 3.4 - Give IUPAC names for the following compounds:Ch. 3.4 - Prob. 12PCh. 3.4 - Name the eight 5-carbon alkyl groups you drew in...Ch. 3.4 - Give the IUPAC name for the following hydrocarbon,...Ch. 3.7 - Make a graph of potential energy versus angle of...Ch. 3.7 - Sight along the C2-C1 bond of 2-methylpropane...Ch. 3.7 - Sight along the C2-C3 bond of 2,3-dimethylbutane,...Ch. 3.7 - Draw a Newman projection along the C2-C3 bond of...Ch. 3.SE - Prob. 19VCCh. 3.SE - Prob. 20VCCh. 3.SE - Draw a Newman projection along the C2-C3 bond of...Ch. 3.SE - Prob. 22APCh. 3.SE - Prob. 23APCh. 3.SE - Propose structures for the following: (a) A...Ch. 3.SE - Prob. 25APCh. 3.SE - Draw the structures of the following molecules:...Ch. 3.SE - Draw structures that meet the following...Ch. 3.SE - Prob. 28APCh. 3.SE - In each of the following sets, which structures...Ch. 3.SE - There are seven constitutional isomers with the...Ch. 3.SE - Prob. 31APCh. 3.SE - Draw compounds that contain the following: (a) A...Ch. 3.SE - Prob. 33APCh. 3.SE - Draw and name all monochloro derivatives of...Ch. 3.SE - Draw structures for the following: (a)...Ch. 3.SE - Prob. 36APCh. 3.SE - Draw a compound that: (a) Has nine primary...Ch. 3.SE - Give IUPAC names for the following compounds:Ch. 3.SE - Name the five isomers of C6H14.Ch. 3.SE - Explain why each of the following names is...Ch. 3.SE - Prob. 41APCh. 3.SE - Consider 2-methylbutane (isopentane). Sighting...Ch. 3.SE - What are the relative energies of the three...Ch. 3.SE - Construct a qualitative potential-energy diagram...Ch. 3.SE - Prob. 45APCh. 3.SE - Draw the most stable conformation of pentane,...Ch. 3.SE - Draw the most stable conformation of...Ch. 3.SE - Prob. 48APCh. 3.SE - Prob. 49APCh. 3.SE - Formaldehyde, H2C=O, is known to all biologists...Ch. 3.SE - Prob. 51APCh. 3.SE - Increased substitution around a bond leads to...Ch. 3.SE - Prob. 53APCh. 3.SE - In the next chapter we'll look at...Ch. 3.SE - We’ll see in the next chapter that there are two...
Knowledge Booster
Similar questions
- 3-39 The following names are all incorrect or incomplete, but they represent real structures. Draw each structure and name it correctly. (a) 2-ethylpentane (d) 2-dimethylbutane (b) 3-isopropylhexane (e) 2-cyclohexylbutane (c) 5-chloro-4-methylhexane (f) 2,3-diethylcyclopentanearrow_forward• -- H (b) H,C 3-methylhexane CH, (c) B கூ CH, CH, HgCG; சீகூ H₂CH H 3-ethyl-1-methyloctane 3,3 diethyl pentane 5Å, Å,CH, W CH3 CH2 CH₂ W21 Su2... CH3 X -CH3 G b F E L carrow_forward- The Newman projections of 1,1-dichloro-2-bromoethane are shown. H H CI [] B 0 A 00 H A C [] D Br Select the most stable conformation(s). CI H Br B CI Br CI Brarrow_forward
- न A 8-£ Barrow_forwardWhich of the following molecules are isomers? (Make sure you can see all of all three structures.) Η Η Η Η | | | H-C-C-C-C-CH III Η Η Η Η I H Ο 1) I and III Ο 2) I and Il Ο 3) II and III 4) I, II, and II Η Η Η H-C-C-C-OH Η Η Η II 5) None of the molecules are isomers Η Η ΟΗΗ ||| I H-C-C-C-C-H || I Η Η Η Η IIIarrow_forwardIncreased substitution around a bond leads to increased strain. Take the four substituted butanes listed below, for example. For each compound, sight along the C2-C3 bond and draw Newman projections of the most stable and least stable conformations. Use the data in Table 3-5 to assign strain-energy values to each conformation. Which of the eight conformations is most strained? Which is least strained? (a) 2-Methylbutane (b) 2,2-Dimethylbutane (c) 2,3-Dimethylbutane (d) 2,2,3-Trimethylbutanearrow_forward
- If you examine the structural formulas for the following cycloalkenes, you will see that the configuration of the double bond is cis in each. Cyclopentene Cyclohexene Cycloheptene All attempts to synthesize these cycloalkenes in which the double bond has a trans configuration have failed. Apparently, it is impossible to have a trans configuration in these cycloalkenes. Offer an explanation for why this is so.arrow_forward11-32 Calculate the actual C-C-C bond angles in planar (a) cyclopropane and (b) cyclopentane and com pare them with optimal bond angles.arrow_forward10-20 Why are the following molecular formulas impossible? (a) CH5 C2H7arrow_forward
- 9. Rank the C-H bond strength of the indicated hydrogen atoms in the following molecules (strongest to weakest C-H bond): B H. C =C _H ii 10. Rank the relative stability of the most stable conformation that the following cyclohexane isomers can adopt (most stable isomer to least stable isomer): B i iiarrow_forwardÁll of the following conformational models are representative of 2,3-dimethylpentane. Which of these models corresponds to its S isomer? Me H Et Me H. Ме Et H Me Me H. Me Me H H H -iPr Me iPr. H Me Me Harrow_forward8. Write the most stable conformer of the following molecules A and B. Calculate the gauche interactions in each and find the difference in their energy. H H H A H H ď H B H Harrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning