
Orthonitroanaline (an important intermediate in dyes—called fast orange) is formed from the reaction of orthonitrochlorobenzene (ONCB) and aqueous ammonia (see explosion in Figure E13-2.1 in Example 13-2).
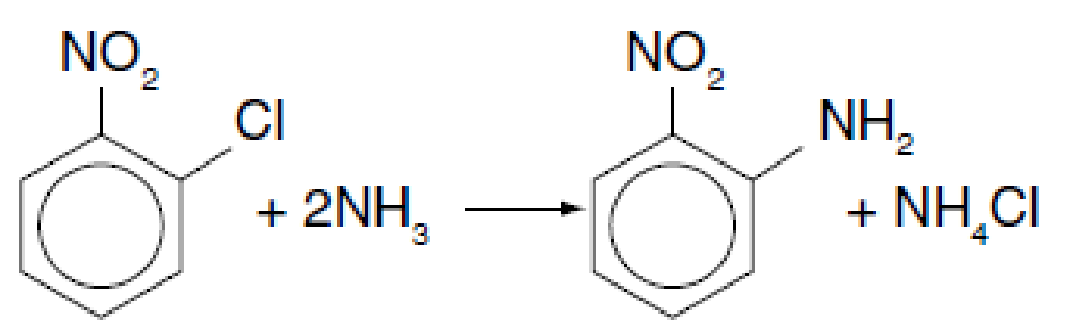
The liquid-phase reaction is first order in both ONCB and ammonia with k = 0.0017 m3/kmol · min at 188°C with E = 11,273 cal/mol. The initial entering concentrations of ONCB and ammonia are 1.8 kmol/m3 and 6.6 kmol/m3, respectively (more on this reaction in Chapter 13).
- (a) Set up a stoichiometric table for this reaction for a flow system.
- (b) Write the rate law for the rate of disappearance of ONCB in terms of concentration.
- (c) Explain how parts (a) and (b) would be different for a batch system.
- (d) Write −rA solely as a function of conversion.
- (e) What is the initial rate of reaction (X = 0)
−rA = ______
at 188°C? −rA = ______
at 25°C? −rA = ______
at 288°C? −rA = ______
- (f) What is the rate of reaction when X = 0.90
at 188°C? −rA = ______
at 25°C? −rA = ______
at 288°C? −rA = ______
- (g) What would be the corresponding CSTR reactor volume at 25°C to achieve 90% conversion and at 288°C for a feed rate of 2 dm3/min
at 25°C? V = ______
at 288°C? V = ______
Want to see the full answer?
Check out a sample textbook solution
Chapter 4 Solutions
Elements of Chemical Reaction Engineering (5th Edition) (Prentice Hall International Series in the Physical and Chemical Engineering Sciences)
Additional Engineering Textbook Solutions
Unit Operations of Chemical Engineering
Essentials of Chemical Reaction Engineering (2nd Edition) (Prentice Hall International Series in the Physical and Chemical Engineering Sciences)
Process Dynamics and Control, 4e
Starting Out with Programming Logic and Design (4th Edition)
EBK FUNDAMENTALS OF THERMODYNAMICS, ENH
Modern Database Management (12th Edition)
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





