
ORGANIC CHEMISTRY W/SSM >CB<
8th Edition
ISBN: 9781323748688
Author: Bruice
Publisher: PEARSON C
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 4.10, Problem 34P
(S)-(+)-Monosodium glutamate (MSG) is a flavor enhancer used in many foods. Some people have an allergic reaction to MSG (including headache, chest pain, and an overall feeling of weakness), fast food often contains substantial amounts of MSG. which is widely used in Chinese food as well (S)-(+)-MSG has a specific rotation of +24.
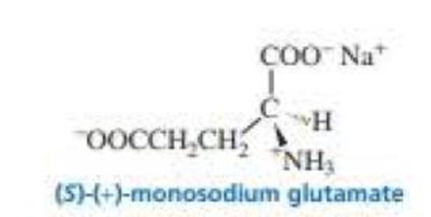
- a. What is the specific rotation of (R)-(–)-monosodium glutamate?
- b. What is the specific rotation of a racemic mixture of MSG?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
(S)-(+)-Monosodium glutamate (MSG) is a flavor enhancer used in many foods. Some people have an allergic reaction to MSG (including headache, chest pain, and an overall feeling of weakness). Fast food often contains substantial amounts of MSG, which is widely used in Chinese food as well. (S)-(+)-MSG has a specific rotation of +24.a. What is the specific rotation of (R)-(-)-monosodium glutamate? b. What is the specific rotation of a racemic mixture of MSG?
The specific rotation of quinine, an anti-malarial drug, is -165°. How much quinine (in %) is present in a sample that
has an observed specific rotation of 33°?
CH3O
HO
H
H
quinine
(antimalarial drug)
The specific rotation of the pure (S) form of Ivermectin is +85.0° when measured using light with a
500 nm wavelength. Ivermectin is an expensive component of a popular COVID treatment called
NO-FX. To save money, the Trumpharm company makes NO-FX by mixing inexpensive racemic
Ivermectin with some enantiomerically pure Ivermectin. A 1.3 g sample of NO-FX is dissolved in
6.0 mL of CHCl3. When placed in a 1 cm cell, the observed rotation at 500 nm is -0.6°.
a) What is the specific rotation of the sample?
b) Which isomer is in excess and how do you know it is in excess?
c) What is the optical purity of the sample?
Chapter 4 Solutions
ORGANIC CHEMISTRY W/SSM >CB<
Ch. 4.1 - Prob. 2PCh. 4.1 - Prob. 3PCh. 4.1 - Prob. 4PCh. 4.1 - Prob. 5PCh. 4.1 - Which of the roll owing compounds have a dipole...Ch. 4.2 - Draw and label the E and Z isomers for each of the...Ch. 4.2 - Assign relative priorities to each set of...Ch. 4.2 - Tamoxifen slows the growth of some breast tumors...Ch. 4.2 - Prob. 10PCh. 4.2 - Name each of the following:
Ch. 4.2 - Draw the Z isomer of an alkene that has a CH3 and...Ch. 4.3 - Prob. 13PCh. 4.4 - Prob. 14PCh. 4.5 - Prob. 16PCh. 4.6 - Prob. 17PCh. 4.7 - Prob. 18PCh. 4.8 - Prob. 20PCh. 4.8 - Prob. 22PCh. 4.8 - Prob. 23PCh. 4.8 - Prob. 24PCh. 4.8 - Draw a perspective formula for each or the...Ch. 4.8 - Prob. 27PCh. 4.9 - Prob. 28PCh. 4.9 - What is the configuration of the following...Ch. 4.9 - Prob. 32PCh. 4.10 - Prob. 33PCh. 4.10 - (S)-(+)-Monosodium glutamate (MSG) is a flavor...Ch. 4.11 - Prob. 35PCh. 4.11 - Prob. 36PCh. 4.12 - Prob. 38PCh. 4.12 - Prob. 39PCh. 4.12 - The stereoisomer of cholesterol found in nature is...Ch. 4.12 - Prob. 41PCh. 4.13 - 1-Bromo-2-methylcyclopentane has four pairs of...Ch. 4.13 - Prob. 43PCh. 4.13 - Draw all possible stereoisomers for each of the...Ch. 4.13 - Prob. 45PCh. 4.13 - Of all the possible cyclooctanes that have one...Ch. 4.13 - Prob. 47PCh. 4.13 - Prob. 48PCh. 4.14 - Which of the following compounds has a...Ch. 4.14 - Draw all the stereoisomers for each of the...Ch. 4.15 - Prob. 52PCh. 4.15 - Name the isomers you drew in Problem 52.Ch. 4.15 - Chloramphenicol is a broad-spectrum antibiotic...Ch. 4.15 - Draw a perspective formula for each of the...Ch. 4.15 - Name the following:Ch. 4.15 - Prob. 57PCh. 4.15 - Prob. 59PCh. 4.15 - Convert the perspective formula to a skeletal...Ch. 4.15 - Prob. 62PCh. 4.16 - Prob. 63PCh. 4.17 - Limonene exists as two different stereoisomers....Ch. 4 - a. Draw three constitutional isomers with...Ch. 4 - Prob. 65PCh. 4 - Prob. 66PCh. 4 - Which of the following has an asymmetric center?...Ch. 4 - Prob. 68PCh. 4 - Prob. 69PCh. 4 - Prob. 70PCh. 4 - Prob. 71PCh. 4 - Assign relative priorities to each set of...Ch. 4 - Prob. 73PCh. 4 - Which of the following are optically active?Ch. 4 - Prob. 75PCh. 4 - Name the following:Ch. 4 - Which of the following has an achiral...Ch. 4 - Using skeletal structures, draw the stereoisomers...Ch. 4 - Prob. 79PCh. 4 - Citrate synthase, one of the enzymes in the series...Ch. 4 - Prob. 81PCh. 4 - Prob. 82PCh. 4 - Prob. 83PCh. 4 - Prob. 84PCh. 4 - Prob. 85PCh. 4 - Prob. 86PCh. 4 - Prob. 87PCh. 4 - Prob. 88PCh. 4 - Prob. 89PCh. 4 - a. Draw all the isomers with molecular formula...Ch. 4 - Prob. 91PCh. 4 - Prob. 92PCh. 4 - Draw structures for the following: a....Ch. 4 - For each of the following structures, draw the...Ch. 4 - Prob. 95PCh. 4 - Prob. 96PCh. 4 - Prob. 97PCh. 4 - a. Using the wedge-and-dash notation, draw the...Ch. 4 - Prob. 99PCh. 4 - Prob. 100PCh. 4 - Prob. 101PCh. 4 - a. Draw the two chair conformers for each of the...Ch. 4 - Prob. 103PCh. 4 - Is the following compound optically active?Ch. 4 - Prob. 105P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The specific rotation, [a]p, for (-)-malic acid is -27. What is the observed rotation for a solution of 0.75 g of (-)-malic acid in 10 mL of water in a sample tube having a pathlength of 10 cm? degrees. The observed rotation of a solution of 1.4 g of a compound in 10 mL of water is +1.3 degrees. If the pathlength is 10 cm, what is the specific rotation of the compound? 9 more group attempts remaining Submit Answer Retry Entire Grouparrow_forwardIf (S)-glyceraldehyde has a specific rotation of -8.7 what is the specific rotation of (R)- glyceraldehyde? O A) -8.7° O B) 0.0° OC) +8.7° O D) cannot be determined from the information givenarrow_forwardThe specific rotation of vitamin B7 in water (22°C) is +92°. A mixture of vitamin B7 and its enantiomer has a specific rotation of + 27.6°. What is the percentage of vitamin B7 and its enantiomer in this mixture? Select one: O A. 60% vitamin B7 : 40% enantiomer B. 65% vitamin B7 : 35% enantiomer C. 55% vitamin B7 : 45% enantiomer D. 70% vitamin B7 : 30% enantiomerarrow_forward
- Glutamic acid is a naturally occurring amino acid and is the precursor to the food additive MSG (monosodium glutamate) which can cause allergic reactions in some people. If naturally occurring L glutamic acid has a melting point of 205 C and an optical rotation of + 31.5, what is the melting point and optical rotation of its enantiomer?arrow_forwardPrectice q #12 The specific rotation of the pure (S) form of effluvium is -25.0°. Efflivium is an expensive component of a popular natural health product called Snake Oil. To save money, the ACME drug company make Snake Oil by mixing inexpensive racemic effluvium with some enantiomerically pure effluvium. The resulting drug gives a specific rotation of+16° a) What is the enantiomeric excess of the sample? b) Which isomer is in excess and why? c) What is the composition of the mixture (how much R form and how much S form)? d) What was the original ratio used of racemic effluvium to enantiomerically pure effluvium?arrow_forwardFind the specific optical rotation of (S)-malic acid having an observed rotation of +15. 5° at a concentration of 5.5 g/ mL in a 1.5 dm solvent at 20°C at a wavelength of 589 nm? O a. 1.25° O b. 2.54° O c. 1.87° O d. 3.45°arrow_forward
- 30. How many chirality centers exist on the following molecule? A. 4 B. 5 C. 6 D. 7arrow_forwardThe specific rotation of vitamin B7 in water (at 22°C) is +92. A chemist prepared a mixture of vitamin B7 and its enantiomer, and this mixture had a specific rotation of +18. Calculate the % ee of this mixture.arrow_forwardThe specific rotation for compound D is -8.5° dm 1(g/mL)-1, what is the specific rotation of compound E under the same conditions? [a] = -8.5° dm-1 (g/mL)-1 OH O он О он ОН D E -8.5° dm-1 (g/mL)-1 +8.5° dm-1 (g/mL)-1 0° dm-1 (g/mL)-1 not enough information is provided to determine a valuearrow_forward
- View Policies Current Attempt in Progress When 0.234 g of monosodium glutamate (MSG) is dissolved in 10.0 mL of water and placed in a sample cell 10.0 cm in length, the observed rotation at 20°(using the D line of sodium) is +0.599°. Calculate the specific rotation of MSG. specific rotation = [a] = i eTexthook and Mediaarrow_forwardThe specific rotation, [a]p, for (-)-malic acid is -27. What is the observed rotation for a solution of 1.2 g of (-)-malic acid in 10 mL of water in a sample tube having a pathlength of 10 cm? | degrees. The observed rotation of a solution of 1.2 g of a compound in 10 mL of water is +0.75 degrees. If the pathlength is 10 cm, what is the specific rotation of the compound? 9 more group attempts remaining Submit Answer Retry Entire Grouparrow_forwardThe specific rotation of S-Ibuprofen is +54.5o. Which direction would a mixture containing 80% S-Ibuprofen and 10% R-ibuprofen rotate plane polarized light? To the left or to the right?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License