
Essential Organic Chemistry, Global Edition
3rd Edition
ISBN: 9781292089034
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 4.2, Problem 9P
Draw and label the E and Z isomers for each of the following:
- a. CH3CH2CH9CHCH3
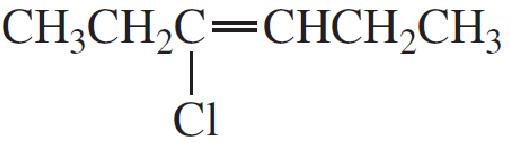
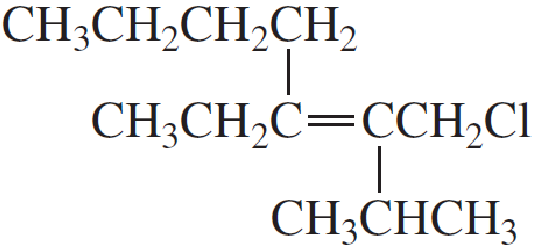
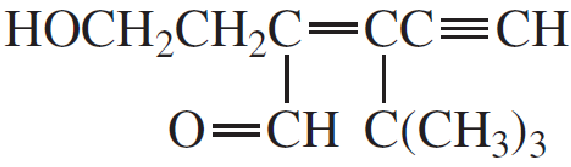
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
https://drive.google.com/file/d/14UPPDWhJXm0KKcLAOXDyBULvI_sISzDW/view?usp=sharing
Which hazard statement is false in regards to dichloromethane?
1) May be corrosive to metals
2) Causes skin irritation
3) Causes serious eye irritation
4) Suspected of causing cancer
The melting points and boiling points of two isomeric alkanes are asfollows: CH3(CH2)6CH3, mp = −57 °C and bp = 126 °C; (CH3)3CC(CH3)3,mp = 102 °C and bp = 106 °C.
Explain why one isomer has a lower melting point but higher boiling point.
What is the chemical formula of the following compound?
Select one:
a.
C4H8O
b.
C3H8O
c.
C4H9O
d.
C3H6C3H8OO
e.
C3H7OH
Chapter 4 Solutions
Essential Organic Chemistry, Global Edition
Ch. 4.1 - Draw the cis and trans isomers for the following:...Ch. 4.1 - Prob. 4PCh. 4.1 - Prob. 5PCh. 4.1 - Prob. 6PCh. 4.2 - Prob. 7PCh. 4.2 - Tamoxifen slows the growth of some breast tumors...Ch. 4.2 - Draw and label the E and Z isomers for each of the...Ch. 4.2 - Prob. 10PCh. 4.2 - Name each of the following:Ch. 4.2 - Draw the structure of (Z)-2,3-dimethyl-3-heptene.
Ch. 4.3 - Prob. 13PCh. 4.4 - Prob. 14PCh. 4.5 - Prob. 16PCh. 4.6 - Prob. 17PCh. 4.7 - Assign relative priorities to the groups or atoms...Ch. 4.7 - Name the following:Ch. 4.7 - Prob. 22PCh. 4.7 - Draw a perspective formula for each of the...Ch. 4.8 - Prob. 24PCh. 4.8 - Prob. 27PCh. 4.9 - Prob. 28PCh. 4.9 - Prob. 29PCh. 4.9 - Prob. 30PCh. 4.10 - Prob. 31PCh. 4.10 - Prob. 32PCh. 4.10 - Prob. 33PCh. 4.11 - Prob. 34PCh. 4.11 - a. Draw the stereoisomers of...Ch. 4.11 - Prob. 37PCh. 4.11 - Prob. 38PCh. 4.12 - Which of the following compounds has a...Ch. 4.12 - Draw all the stereoisomers for each of the...Ch. 4.12 - Limonene exists as two different stereoisomers....Ch. 4 - a. Draw three constitutional isomers with...Ch. 4 - Which of the following have an asymmetric center?...Ch. 4 - Prob. 45PCh. 4 - Prob. 46PCh. 4 - Of all the possible cyclooctanes that have one...Ch. 4 - Prob. 48PCh. 4 - Prob. 49PCh. 4 - Prob. 50PCh. 4 - Prob. 51PCh. 4 - Prob. 52PCh. 4 - Draw the stereoisomers of 2,4-dichlorohexane....Ch. 4 - Prob. 54PCh. 4 - Prob. 55PCh. 4 - Prob. 56PCh. 4 - Prob. 57PCh. 4 - Prob. 58PCh. 4 - Prob. 59PCh. 4 - Prob. 60PCh. 4 - Prob. 61PCh. 4 - Prob. 62PCh. 4 - Draw structures for each of the following: a....Ch. 4 - Prob. 64PCh. 4 - a. Draw all the isomers with molecular formula...Ch. 4 - Prob. 66PCh. 4 - Prob. 67PCh. 4 - Prob. 68PCh. 4 - Chloramphenicol is a broad-spectrum antibiotic...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the 3D representation of these chemicals BeCl2 NCl3 BrCl3 BrF5arrow_forwardPlease draw the Z and E isomers of 1,3-dichloro-2-methylbut-2-ene Thank youarrow_forwardGiven the molecular structure of Caprazamycin B (CPB, figure 1), answer the following questions. Figure 1.1 Structure of Caprazamycin B (CPB). 1.1 How many atoms with sp2 hybridisation does CPB have? 1.2 List the functional groups present in CPB.1.3 How many chiral centres does CPB have?1.4 How many different diasteroisomers can CPB have?arrow_forward
- what is the structure and jvalues? (c10H14O).arrow_forward1. Name and draw the isomers form from the given molecular formula. 20 pts. a. C4H10 b. C4H8Br2arrow_forwardWhich of the following substances would be expected to have the lowest melting point ? A) CH3CH2CH2CH2CH3 B) CH3CH3 C) CH3CH2CH2CH3 D) CH4 E) CH3CH2CH3arrow_forward
- Draw a three-dimensional representation (using wedges and dashed lines) of the structure.(a) CO2 (b) CH3OCH3 (c) (CH3)3O+ (d) CH3COOH(e) CH3CCH (f) CH3CHNCH3 (g) H2CCOarrow_forwardWhat are missing isomers and hydribizationarrow_forwardDraw 3 isomers that have the molecular formula of C5H7Br2NYou may want to calculate HDI first.arrow_forward
- Determine the reagent that could best differentiate the two compounds given. A. ZnCl2 B. NaOH C. FeCl3 D. KMnO4arrow_forwardWhat is a hydrocarbon? What is the difference between a saturated hydrocarbon and an unsaturated hydrocarbon? Distinguish between normal and branched hydrocarbons. What is an alkane? What is a cyclic alkane? What are the two general formulas for alkanes? What is the hybridization of carbon atoms in alkanes? What are the bond angles in alkanes? Why are cyclopropane and cyclobutane so reactive? The normal (unbranched) hydrocarbons are often referred to as straight-chain hydrocarbons. What does this name refer to? Does it mean that the carbon atoms in a straight-chain hydrocarbon really have a linear arrangement? Explain. In the shorthand notation for cyclic alkanes, the hydrogens are usually omitted. How do you determine the number of hydrogens bonded to each carbon in a ring structure?arrow_forwardmethods to differentiate the isomers in between [RhCl2(H2O)4]Cl and [RhCl3(H2O)3].H2Oarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY