
Concept explainers
Interpretation:

The IUPAC names of the given compounds are to be determined.
Concept introduction:
The halogen is treated as substituent on an
The two substituents are considered of equal rank, when both halogen and an alkyl substituent are on the same carbon chain. The substituent nearer the end of the chain is given the lower number.
Answer to Problem 20P
Solution:
Explanation of Solution
(a)
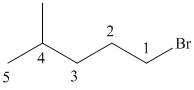
In this structure, the longest carbon chain is “pentane,” which contains
Therefore, the IUPAC name of the given structure is
(b)
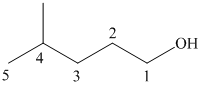
In this structure, the longest carbon chain is “pentane,” which contains
Therefore, the IUPAC name of given structure is
(c)
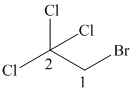
In this structure, the longest carbon chain is “ethane,” which contains
Therefore, the IUPAC name of given structure is
(d)
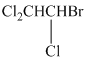
The given compound is converted to the structural formula as shown below:
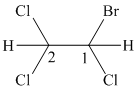
In the given structure, the longest possible parent chain is “ethane,” which contains
Therefore, the IUPAC name of given structure is
(e)
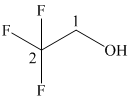
In the given structure, the longest possible parent chain is “ethane,” which contains
(f)
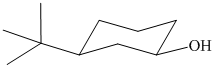
In the given structure, the parent chain is cyclic and has six carbon atoms. Hence the parent alkane is cyclohexane. One carbon atom is attached to a hydroxyl group. Hydroxyl groups take precedence over alkyl groups in determining the direction in which a carbon chain is numbered. Hence, the carbon atom attached to the hydroxyl group is numbered
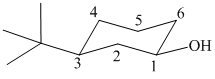
Thus, the IUPAC name of the compound is
(g)
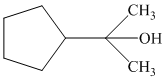
In the given structure, a five member cyclopentane ring is present. There is a three carbon atom chain bearing the hydroxyl group. The propane chain becomes the parent since the hydroxyl group is attached to it. The cyclopentane ring is considered as substituent and attached to the second carbon of the propane chain as shown:
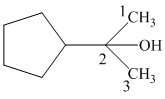
Thus, the IUPAC name of the given structure is
(h)
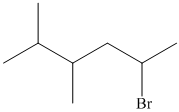
In the given structure, the longest possible parent chain is “Hexane,” which contains
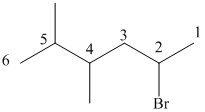
The prefix di is used to indicate the presence of two methyl groups.
Thus, the IUPAC name of the given structure is
(i)
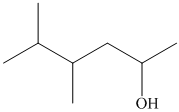
In the given structure, the longest possible parent chain is “Hexane,” which contains
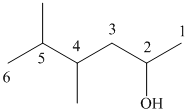
Thus, the IUPAC name of the compound is
Want to see more full solutions like this?
Chapter 5 Solutions
ORGANIC CHEMISTRY-W/STUD.SOLN.MAN.
- Step 1c: Draw the structure(s) resulting from the curved arrow mechanism of step 1. Include formal charges and all hydrogen atoms, but do not include lone pairs.arrow_forwardHydrocarbon A possesses a significant dipole, even though it iscomposed of only C—C and C—H bonds. Explain why the dipole arisesand use resonance structures to illustrate the direction of the dipole.Which ring is more electron rich?arrow_forwardComplete the reaction map by providing the answer from A-E. Write the IUPAC name of the products. conc HNO B conc H,SO, B, D FeBrs conc HNO, E AIC conc H2SO. conc H2SO4arrow_forward
- Methyl isocyanate, CH3 -N= C = O, is used in the industrial synthesis of a type of pesticide and herbicide known as a carbamate. As a historical note, an industrial accident in Bhopal, India, in 1984 resulted in leakage of an unknown quantity of this chemical into the air. An estimated 200,000 people were exposed to its vapors, and over 2000 of these people died. Q.) Methyl isocyanate reacts with strong acids, such as sulfuric acid, to form a cation. Will this molecule undergo protonation more readily on its oxygen or nitrogen atom? In considering contributing structures to each hybrid, do not consider structures in which more than one atom has an incomplete octetarrow_forwardfor the following formulas drew 2 constitutional isomers and provide names in IUPAC for ypur drawingsarrow_forward12.4 (j-k)Predict the product and give the name of the reactionarrow_forward
- 7. Name the following Compound: HNO3(aq) Group of answer choices a) Nitric Acid b) Hydrogen Nitrate c) Hydro Nitric Acid d) Hydrogen Nitro Trioxidearrow_forwardWhich of the following molecules has only single bonds. A. CHCHCH3 B. CH2CHCH3 C. CH3CH2CCH D. CH3CH3 E. CH2CH2 Which of the following molecules has a carbon-to-carbon double bond? A. CH3CCH B. CHCH C. CH3CH3 D. CH3CH2CH3 E. CH2CHCH3arrow_forwardA compound C4H11N is known from its reactivity andspectroscopic properties to have no hydrogen atomsattached directly to the nitrogen atom. Write all structuralformulas consistent with this informationarrow_forward
- a. Draw and name all of the isomeric products obtainedfrom the monobromination of propane with Br2/light. If halogenation were a completely randomreaction and had an equal probability of occurring atany of the C—H bonds in a molecule, what percentage of each of these monobromo products would beexpected?b. Answer part (a) using 2-methylpropane as the starting material.arrow_forwardOrder the compounds below from least to most thermodynamically stable (relative to their constituent elements) at 25oC. name formula △Gof(kJmol) dichloromethane CH2Cl2(l) -63.2 hydrazine N2H4(l) +149.4 hydrogen peroxide H2O2(l) -120.4 (least stable) < < (most stable)arrow_forwardCompound ΔHf (kj/mol) C2H5OH(i) -277.6 H2O(i) -285.8 H2O(g) -241.8 CO2(g) -393.5 CO(g) -110.5 H2O2(i) -187.6 C3H8(g) -103.8 What is the ΔH for the following reaction? C2H5OH(i) + 3O2 (g) --> 2CO2(g) + 3H2O(i)arrow_forward

 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

