
Write structural formulas for all the constitutionally isomeric alcohols of molecular
formula
Interpretation:
The structural formulas for the constitutionally isomeric alcohols of molecular formula
Concept Introduction:
Isomers that have the same molecular formula but differ in the way in which different atoms are connected are known as constitutional isomers.
The number of atoms of each element present in the compound, is known as the molecular formula.
When the functional group
Answer to Problem 22P
Solution:
There are 8 constitutionally isomeric alcohols of molecular formula
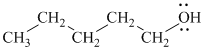
Substitutive name:
Functional class name: pentyl alcohol
It is a primary alcohol.
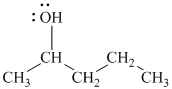
Substitutive name:
Functional class name:
It is a secondary alcohol.
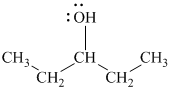
Substitutive name:
Functional class name:
It is a secondary alcohol.
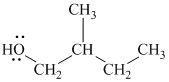
Substitutive name:
Functional class name:
It is a primary alcohol.
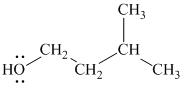
Substitutive name:
Functional class name:
It is a primary alcohol.
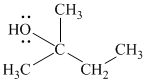
Substitutive name:
Functional class name:
It is a tertiary alcohol.
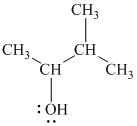
Substitutive name:
Functional class name:
It is a secondary alcohol.
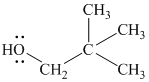
Substitutive name:
Functional class name:
It is a primary alcohol.
Explanation of Solution
In all, there are
They have the same molecular formula but different connectivity of atoms.
The structural formulas of these alcohols can be written as follows.
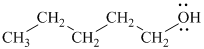
The substitutive name of this alcohol is
The functional class name of this alcohol is pentyl alcohol.
The hydroxyl group is attached to a primary carbon atom. Hence, it is a primary alcohol.
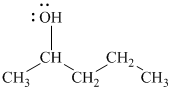
The substitutive name of this alcohol is
The functional class name of this alcohol is
The hydroxyl group is attached to a secondary carbon atom. Hence, it is a secondary alcohol.
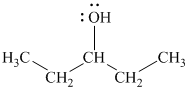
The substitutive name of this alcohol is
The functional class name of this alcohol is
The hydroxyl group is attached to a secondary carbon atom. Hence, it is a secondary alcohol.
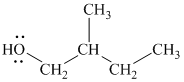
The substitutive name of this alcohol is
The functional class name of this alcohol is
The hydroxyl group is attached to a primary carbon atom. Hence, it is a primary alcohol.
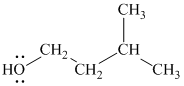
The substitutive name of this alcohol is
The functional class name of this alcohol is
The hydroxyl group is attached to a primary carbon atom. Hence, it is a primary alcohol.
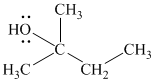
The substitutive name of this alcohol is 2-methyl-2-butanol.
The functional class name of this alcohol is 1,1-dimethylpropanol.
The hydroxyl group is attached to a tertiary carbon atom. Hence, it is a tertiary alcohol.
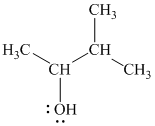
The substitutive name of this alcohol is
The functional class name of this alcohol is
The hydroxyl group is attached to a secondary carbon atom. Hence, it is a secondary alcohol.
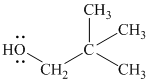
The substitutive name of this alcohol is
The functional class name of this alcohol is
The hydroxyl group is attached to a primary carbon atom. Hence, it is a primary alcohol.
The structural formulas for the constitutionally isomeric alcohols of molecular formula
Want to see more full solutions like this?
Chapter 5 Solutions
ORGANIC CHEMISTRY-PACKAGE >CUSTOM<
- 1. In the reactions involving the three isomeric alcohols with the formula C4H9OH, describewhat each of the following tests showed about reactivity of the -OH group and reactions of 1°,2°, and 3° alcohols.• the test with neutral KMnO4• the test with concentrated HCl2. Predict how the fourth alcohol with the formula C4H10O would react if tested with:• 0.01 M KMnO4• concentrated HCl at room temperatureExtend FurtherUse your observations of the solutions formed in the previous experiments and yourunderstanding of alcohols to complete a table like the one shown below. Research the meltingand boiling points to verify your answers.arrow_forwardDifferentiate: 1. Monhydric from dihydric alcohols. 2.Primary from secondary from tertiary alcohols as to functional group, method of preparation, and on oxidation. 3. Absolute alcohol from refined alcohol. 4. Ethyl alcohol from methyl alcohol.arrow_forwardWrite structural formulas for all ketones with the molecular formula C6H12O and give each its IUPAC name. Which of these ketones are chiral?arrow_forward
- Show how you would use a simple chemical test to distinguish between the followingpairs of compounds. Tell what you would observe with each compound.(a) isopropyl alcohol and tert-butyl alcohol(b) isopropyl alcohol and butan-2-one, CH3COCH2CH3arrow_forwardThere are several isomeric alcohols and ethers of molecular formula C5H12O. Propose a structure for the isomer. Isomer A: δ = 1.19 (s, 9 H), 3.21 (s, 3H) ppmarrow_forwardDraw structural formulas for the alkene that gives each alcohol upon hydroboration-oxidation.arrow_forward
- Propose a structural formula for the product(s) when each of the following alkenes is treated with H2O/H2SO4. Why are two products formed in part (b), but only one in parts (a) and (c)? (a) 1-Hexene gives one alcohol with a molecular for- mula of C6H14O. (b) 2-Hexene gives two alcohols, each with a molecu- lar formula of C6H14O. (c) 3-Hexene gives one alcohol with a molecular for- mula of C6H14O.arrow_forwardArrange the following alcohols in increasing solubility in water ethanol 1-pentanol 1-propanol 1-hexanol a. I < II < III < IV b. IV < II < III < I c. IV > II > III > I d. I > II> III > IVarrow_forwardWrite structural formulas for the major organic product(s) formed by reaction of 1-methylcyclohexene with oxidizing agent. Q.) O3 followed by (CH3)2Sarrow_forward
- The fungus responsible for Dutch elm disease is spread by European bark beetles when they burrow into the tree. Other beetles congregate at the site, attracted by the scent of a mixture of chemicals, some emitted by other beetles and some coming from the tree. One of the compounds given off by female bark beetles is 4-methyl-3-heptanol. Suggest an efficient synthesis of this pheromone from alcohols of five carbon atoms or fewer.arrow_forwardMixing cyclohexanol with phosphoric acid is an exothermic process, whereas the production of cyclohexene is endothermic. Construct an energy diagram showing the course of this reaction. Label the diagram with the starting alcohol, the oxonium ion (the protonated alcohol), the carbocation, and the product.arrow_forward1. (a) Which compound would have the highest boiling point? benzophenone toluene acetophenone benzaldehyde (b) Which of the reagents listed below can convert a primary alcohol to an aldehyde? H2CrO4 DMSO, (COCl)2, Et3N AgNO3 KMnO4-, NaOH (c) Oxidation of alcohols involving the use of DMSO and (COCl)2 is also known as which of the following? Jones Tollens Baeyer-Villiger Swernarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

