
Concept explainers
(a)
Interpretation:
The electrophilic group for the given reaction is to be identified.
Concept introduction:
The replacement or substitution of one functional group with another different functional group in any
Answer to Problem 5.1P
In the given reaction, iodine cation is behaving as an electrophile.
Explanation of Solution
In acid catalyzed hydration reaction, nucleophile goes to the more substituted carbon of the
In the given reaction, addition of electrophile over carbon-carbon double bond takes place. This will result in the formation of intermediate that is carbocation. Thus, the addition of electrophile is at less substituted carbon of the alkene to give the desired product as shown below.
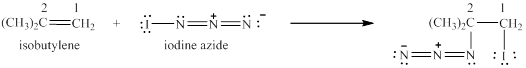
Figure 1
In this case, azide ion is behaving as a nucleophile, whereas iodine cation is behaving as an electrophile.
In the given reaction, iodine cation is behaving as an electrophile.
(b)
Interpretation:
The product formed by the given reaction is to be stated.
Concept introduction:
The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron rich chemical species that contains negative charge or lone pair of electrons are known as a nucleophile. In a nucleophilic substitution reaction, nucleophile takes the position of leaving group by attacking the electron deficient carbon atom.
Answer to Problem 5.1P
The product formed by the given reaction is shown below.
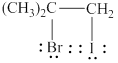
Explanation of Solution
The given reaction is,
![]()
Figure 2
The electronegativity of bromine is higher than iodine, thus due to which in iodine bromide iodine will act as an electrophile, whereas bromide ion will act as a nucleophile. The given reaction will follow Markovnikov’s rule to form the desired product as shown below.

Figure 3
The product formed by the given reaction is shown in Figure 3.
Want to see more full solutions like this?
Chapter 5 Solutions
ORGANIC CHEMISTRY (LL)+ SAPLING ACC >BI
- One frequently used method for preparing methyl esters is by reaction of carboxylic acids with diazomethane, CH2N2. The reaction occurs in two steps: (l) protonation of diazomethane by the carboxylic acid to yield methyldiazonium ion, CH3N2+, plus a carboxylate ion; and (2) reaction of the carboxylate ion with CH3N2+. (a) Draw two resonance structures of diazomethane, and account for step 1. (b) What kind of reaction occurs in step 2?arrow_forwardFollowing is a balanced equation for bromination of toluene. (a) Using the values for bond dissociation enthalpies given in Appendix 3, calculate H0 for this reaction. (b) Propose a pair of chain propagation steps and show that they add up to the observed reaction. (c) Calculate H0 for each chain propagation step. (d) Which propagation step is rate-determining?arrow_forwardA compound X of molecular formula C7H14 when hydrogenated produces 2,4-dimethylpentane and when hydroborated the obtained alcohol is 2,4-dimethyl-1-pentanol. a. What is the structure of X? b. If X is reacted with H2O catalyzed by H2SO4, what product is obtained? Write the structure, give the name, and write the complete mechanism, step by step of this reaction, in such a way as to explain the formation of the compound (DO NOT FORGET THE CORRECT USE OF ARROWS!).arrow_forward
- Use benzenonium resonance forms to explain why the methoxyl group (-OCH3) is o, p-directing in electrophilic aromatic substitution reactions.arrow_forwardDraw a resonance structure of the acetonitrile anion, :CH2C =N, and account for the acidity of nitriles.arrow_forwardAlkyl halides can be reduced to alkanes by a radical reaction with tributyltin hydride, (C4H9)3SnH, in the presence of light (hv). Propose a radical chain mechanism by which the reaction might occur. The initiation step is the light-induced homolytic cleavage of the Sn-H bond to yield a tributyltin radical.arrow_forward
- Propose a reasonable mechanism for the reaction, drawing out Lewis structure, using arrows to show electron flow, clearly identifying the bond making/bond breaking steps, and proposing reasonable intermediates for the reaction.arrow_forwardIn the reaction of ethylene with H20O in the presence of sulfuric acid,which one adds across the double bond first? *arrow_forwardDraw the structure of each product from the reaction of benzene with 2-chloro-1-methylcyclohexane using AlCl 3 as the catalyst and Identify the major product.arrow_forward
- Explain why methyl trifluoroacetate, CF3CO2CH3, is more reactive than methyl acetate, CH3CO2CH3, in nucleophilic acyl substitution reactions.arrow_forwardIsoprene has sometimes been used as a starting material in the laboratory synthesis of terpenes. In one such synthesis, the first step is the electrophilic addition of 2 mol of hydrogen bromide to isoprene to give 1,3-dibromo-3-methylbutane.Write a series of equations describing the mechanism of this reaction.arrow_forwardProvide mechanisms for this reactionarrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning


