
(a)
Interpretation:
The graph of diffusion coefficient (D) m2/s, v/s inverse of temperature (1/T) needs to be plotted using the given data.
Concept Introduction:
Diffusion coefficient (D) is given by the following relation:
Where,
Answer to Problem 5.44P
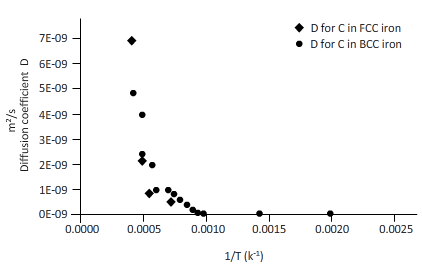
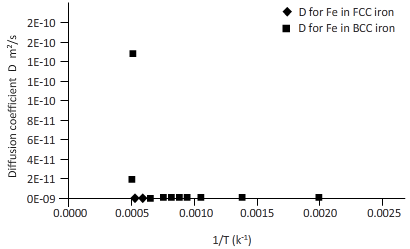
The curves between diffusion coefficient and inverse temperature for Fe atom in FCC and BCC iron are as follows:
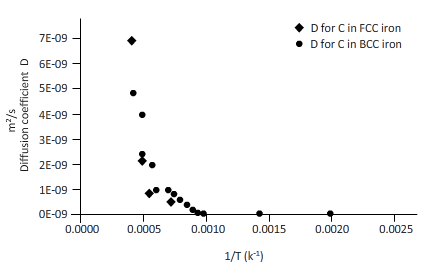
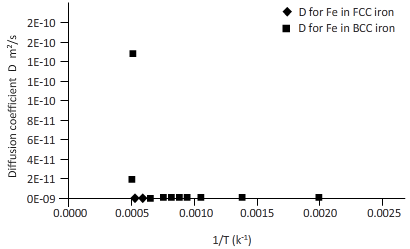
Explanation of Solution
Given Information:
| Diffusion couple | Diffusion | Q(J/mol) | Do(m2/s) |
| C in FCC iron | Interstitial | ||
| C in BCC iron | Interstitial | ||
| Fe in FCC iron | vacancy | ||
| Fe in BCC iron | vacancy |
Calculation:
The diffusion coefficient can be calculated as follows:
Here,
Equation (1) becomes
Hence, coefficient of diffusion for C in FCC iron at
Now, draw a table to calculate the coefficient of diffusion for C in FCC iron and for C in BCC iron diffusion temperature.
| T(k) | 1/T(k-1) | D for C in FCC iron ( | D for C in BCC iron ( |
| 2000 | 0.0005 | 6E-09 | 6E-09 |
| 1750 | 0.0006 | 2E-09 | 3E-09 |
| 1500 | 0.0007 | 4E-10 | 1E-09 |
| 1250 | 0.0008 | 4E-11 | 2E-10 |
| 1200 | 0.0008 | 2E-11 | 2E-10 |
| 1150 | 0.0009 | 1E-11 | 1E-10 |
| 1100 | 0.0009 | 7E-12 | 8E-11 |
| 1000 | 0.0010 | 1E-12 | 3E-11 |
| 750 | 0.0013 | 6E-15 | 9E-13 |
| 500 | 0.0020 | 9E-20 | 8E-16 |
From the table, plot a curve between inverse temperature (1/T) and diffusion coefficient D.
Again draw a table to calculate the coefficient of diffusion for Fe in FCC iron and for Fe in BCC iron at different temperature.
| T(k) | 1/k(k-1) | D for C in FCC iron ( | D for C in BCC iron ( |
| 2000 | 0.0005 | 3E-12 | 2E-10 |
| 1750 | 0.0006 | 3E-13 | 2E-11 |
| 1500 | 0.0007 | 1E-14 | 1E-12 |
| 1250 | 0.0008 | 1E-16 | 2E-14 |
| 1200 | 0.0008 | 5E-17 | 8E-15 |
| 1150 | 0.0009 | 1E-17 | 3E-15 |
| 1100 | 0.0009 | 4E-18 | 9E-16 |
| 1000 | 0.0010 | 2E-19 | 6E-17 |
| 750 | 0.0013 | 2E-24 | 3E-21 |
| 500 | 0.0020 | 5E-34 | 9E-30 |
Plotting the curve between inverse temperature (1/T) and diffusion coefficient (D) for Fe atom in FCC and BCC.
(b)
Interpretation:
The temperature range at which the iron-transition takes place needs to be determined from the graphs.
Concept Introduction:
Iron transition is the period in which a subject change from one state to another takes place.
Answer to Problem 5.44P
The iron transition for BCC to FCC takes place in a range of temperature 1150 K-1200 K.
The iron transition for FCC to BCC takes place in the range of 1600 K-1700 K.
Explanation of Solution
Given Information:
| Diffusion couple | Diffusion mechanism | Q(J/mol) | Do(m2/s) |
| C in FCC iron | Interstitial | ||
| C in BCC iron | Interstitial | ||
| Fe in FCC iron | vacancy | ||
| Fe in BCC iron | vacancy |
The curves between diffusion coefficient and inverse temperature for Fe atom in FCC and BCC iron are as follows:
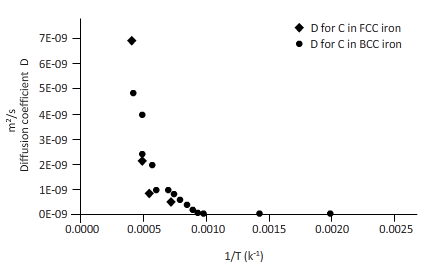
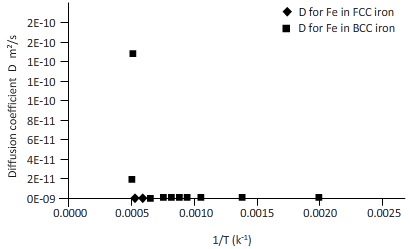
From the curve (1),
BCC iron to FCC iron transition takes place in a range of temperature 1150 K-1200 K.
From the curve (2),the iron transition from BCC to FCC iron take place in the temperature range 1600 K-1700 K.
(c)
Interpretation:
The reason for the higher activation energy for diffusion in FCC iron than carbon needs to be explained.
Concept Introduction:
Activation energy, D is related to the size of the atom.
Answer to Problem 5.44P
An atomic number of carbon (C) is 6 while the atomic number of iron (Fe) is 26.
As we know that activation is related to the size of atom hence more atomic number greater is the activation energy required.
Explanation of Solution
Diffusion atom is placed in a vacancy position.
But in some atoms, there is a defect due to more interstitial position and less vacancy. Diffusion atom takes less time to reach in interstitial position than vacancy. Activation energy co-relates with atomic number. Hence, more atomic number, maximum activation energy is required. The atomic number of carbon is 4 and that of iron is 26. Thus, the activation energy for diffusion of Fe is higher than that of C.
(d)
Interpretation:
The reason for the higher activation energy for the diffusion of FCC than BCC iron needs to be explained.
Concept Introduction:
Activation energy is directly proportional to the packing fraction. Packing fraction is a bond formed by atoms.
Answer to Problem 5.44P
Packing fraction of FCC structure is 0.71 while that for the BCC structure is 0.68.
Hence, the activation energy required for FCC is more than BCC.
Explanation of Solution
Packing fraction is the measurement of loss or gain of the total mass in a group of the nucleus which directly relates atomic bond. Packing fraction is correlated with activation energy. The packing fraction for FCC structure is 0.74 and that for the BCC structure is 0.68.
The activation energy for diffusion higher in FCC as compared to the BCC iron.
(e)
Interpretation:
Whether the diffusion rate in FCC is faster than in BCC or not needs to be determined.
Concept Introduction:
Diffusion coefficient (D) is given by the following relation:
Where,
Answer to Problem 5.44P
Diffusion rate in FCC structure is slower than the BCC structure.
Explanation of Solution
Taking T= 1175 K from temperature range of BCC to FCC iron transition.
Since,
Substitute,
Equation (1) becomes,
Again to find out 'D' in BCC iron for carbon.
Put
Equation (1) becomes,
From (a) and (b), one can say that the diffusion coefficient is more in BCC structure than the FCC.
Hence, diffusion atoms move faster in BCC structure compared to FCC structure.
Want to see more full solutions like this?
Chapter 5 Solutions
ESS.MAT.SCI (LL W/MINDTAP)
 MATLAB: An Introduction with ApplicationsEngineeringISBN:9781119256830Author:Amos GilatPublisher:John Wiley & Sons Inc
MATLAB: An Introduction with ApplicationsEngineeringISBN:9781119256830Author:Amos GilatPublisher:John Wiley & Sons Inc Essentials Of Materials Science And EngineeringEngineeringISBN:9781337385497Author:WRIGHT, Wendelin J.Publisher:Cengage,
Essentials Of Materials Science And EngineeringEngineeringISBN:9781337385497Author:WRIGHT, Wendelin J.Publisher:Cengage, Industrial Motor ControlEngineeringISBN:9781133691808Author:Stephen HermanPublisher:Cengage Learning
Industrial Motor ControlEngineeringISBN:9781133691808Author:Stephen HermanPublisher:Cengage Learning Basics Of Engineering EconomyEngineeringISBN:9780073376356Author:Leland Blank, Anthony TarquinPublisher:MCGRAW-HILL HIGHER EDUCATION
Basics Of Engineering EconomyEngineeringISBN:9780073376356Author:Leland Blank, Anthony TarquinPublisher:MCGRAW-HILL HIGHER EDUCATION Structural Steel Design (6th Edition)EngineeringISBN:9780134589657Author:Jack C. McCormac, Stephen F. CsernakPublisher:PEARSON
Structural Steel Design (6th Edition)EngineeringISBN:9780134589657Author:Jack C. McCormac, Stephen F. CsernakPublisher:PEARSON Fundamentals of Materials Science and Engineering...EngineeringISBN:9781119175483Author:William D. Callister Jr., David G. RethwischPublisher:WILEY
Fundamentals of Materials Science and Engineering...EngineeringISBN:9781119175483Author:William D. Callister Jr., David G. RethwischPublisher:WILEY





