
(a)
Interpretation:
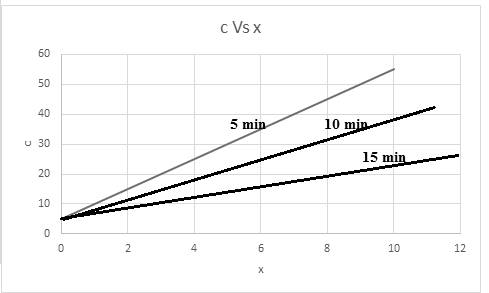
A graph of concentration (c) with x in centimeter at a time for
Concept introduction:
Fick's Law of diffusion: This law states that molar flux is directly proportional to concentration gradient. The law is stated as:
Where,
J is the molar flux defined as the number of atoms passing per unit area per unit time.
D is the diffusion coefficient in
Factors affection diffusion are as follows:
- Temperature
- Diffusion coefficient
The following equation is stated as:
Where,
Q is the activation energy in calorie/ mole.
R is universal gas constant in
T is the absolute temperature in kelvin.
Answer to Problem 5.71P
The required graph for concentration versus x is shown below:
Explanation of Solution
Given information:
The equation used is given as,
Where,
Q is the initial surface concentration having unit of
Temperature for water is
Diffusion coefficient of phosphorous in silicon at a temperature of
t is the time in seconds.
Given equation for finding out the plot between of concentration (c) with x,
Based on given data calculation of
Substituting the values,
The relationship of concentration at a time of 5 min is,
Given equation for finding out the plot between of concentration (c) with x,
Based on given data calculation of
Substituting the values,
The relationship of concentration at a time of 10 min is,
Given equation for finding out the plot between of concentration (c) with x,
Based on given data calculation of
Substituting the values,
The relationship of concentration at a time of 15 min is,
Thus, based on given relation the concentration is dependent on x and t. The graph for the same considering time into consideration,
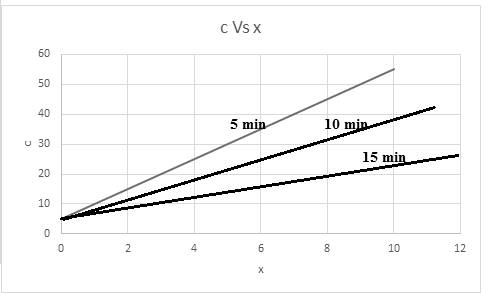
The graph is representing the linear slope of concentration versus x with respect to given time.
(b)
Interpretation:
Time required by the phosphorous concentration to make it equal to the concentration of boron at a depth of
Concept introduction:
Fick's Law of diffusion: This law states that molar flux is directly proportional to concentration gradient. The law is stated as:
Where,
J is the molar flux defined as the number of atoms passing per unit area per unit time.
D is the diffusion coefficient in
Factors affection diffusion are as follows:
- Temperature
- Diffusion coefficient
The following equation is stated as:
Where,
Q is the activation energy in calorie/ mole.
R is universal gas constant in
T is the absolute temperature in kelvin.
Answer to Problem 5.71P
Thus, the value of Time required by the phosphorous concentration to make it equal to the concentration of boron at a depth of
Explanation of Solution
Given information:
The equation used is given as,
Where,
Q is the initial surface concentration having unit of
Temperature for water is
Diffusion coefficient of phosphorous in silicon at a temperature of
t is the time in seconds.
Given equation for finding out the value of time (t) in seconds
Based on given data calculation of
Substituting the values,
On solving the equation,
t = 49758906
Conversion of seconds to hours,
Thus, the value of Time required by the phosphorous concentration to make it equal to the concentration of boron at a depth of
Want to see more full solutions like this?
Chapter 5 Solutions
Essentials Of Materials Science And Engineering
 MATLAB: An Introduction with ApplicationsEngineeringISBN:9781119256830Author:Amos GilatPublisher:John Wiley & Sons Inc
MATLAB: An Introduction with ApplicationsEngineeringISBN:9781119256830Author:Amos GilatPublisher:John Wiley & Sons Inc Essentials Of Materials Science And EngineeringEngineeringISBN:9781337385497Author:WRIGHT, Wendelin J.Publisher:Cengage,
Essentials Of Materials Science And EngineeringEngineeringISBN:9781337385497Author:WRIGHT, Wendelin J.Publisher:Cengage, Industrial Motor ControlEngineeringISBN:9781133691808Author:Stephen HermanPublisher:Cengage Learning
Industrial Motor ControlEngineeringISBN:9781133691808Author:Stephen HermanPublisher:Cengage Learning Basics Of Engineering EconomyEngineeringISBN:9780073376356Author:Leland Blank, Anthony TarquinPublisher:MCGRAW-HILL HIGHER EDUCATION
Basics Of Engineering EconomyEngineeringISBN:9780073376356Author:Leland Blank, Anthony TarquinPublisher:MCGRAW-HILL HIGHER EDUCATION Structural Steel Design (6th Edition)EngineeringISBN:9780134589657Author:Jack C. McCormac, Stephen F. CsernakPublisher:PEARSON
Structural Steel Design (6th Edition)EngineeringISBN:9780134589657Author:Jack C. McCormac, Stephen F. CsernakPublisher:PEARSON Fundamentals of Materials Science and Engineering...EngineeringISBN:9781119175483Author:William D. Callister Jr., David G. RethwischPublisher:WILEY
Fundamentals of Materials Science and Engineering...EngineeringISBN:9781119175483Author:William D. Callister Jr., David G. RethwischPublisher:WILEY





