
Concept explainers
(a)
Interpretation:
Lewis dot symbol for the given atoms/ions must be shown
Concept Introduction:
Lewis dot symbol is used to represent the valence electrons of an atom or ion using dots surrounding the element symbol along four sides of the element symbol without maintaining exact order for the placement of dots.
In Lewis dot symbol representation, the
The valence electron is the number of electrons present in the outermost shell of the atom. The number of valence electrons will be same for the same group elements which is represented by Lewis dot symbol.
(a)
Answer to Problem 5.7QP
Lewis dot representation for
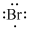
Explanation of Solution
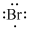
The electronic configuration of Bromine is
(b)
Interpretation:
Lewis dot symbol for the given atoms/ions must be shown
Concept Introduction:
Lewis dot symbol is used to represent the valence electrons of an atom or ion using dots surrounding the element symbol along four sides of the element symbol without maintaining exact order for the placement of dots.
In Lewis dot symbol representation, the symbol of element is surrounded by "dots" indicating the number of valence electrons available for the element. The dots can be placed one at a time on all the four sides, further electron can be placed by pairing up with the first placed dots. According to the number of electrons added or removed, charge must be placed on the Lewis dot symbol for cations and anions.
The valence electron is the number of electrons present in the outermost shell of the atom. The number of valence electrons will be same for the same group elements which is represented by Lewis dot symbol.
(b)
Answer to Problem 5.7QP
Lewis dot representation for
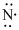
Explanation of Solution
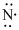
The electronic configuration of Nitrogen is
Lewis dot symbol denotes the valence electron of an atom. The valence electron of
(c)
Interpretation:
Lewis dot symbol for the given atoms/ions must be shown
Concept Introduction:
Lewis dot symbol is used to represent the valence electrons of an atom or ion using dots surrounding the element symbol along four sides of the element symbol without maintaining exact order for the placement of dots.
In Lewis dot symbol representation, the symbol of element is surrounded by "dots" indicating the number of valence electrons available for the element. The dots can be placed one at a time on all the four sides, further electron can be placed by pairing up with the first placed dots. According to the number of electrons added or removed, charge must be placed on the Lewis dot symbol for cations and anions.
The valence electron is the number of electrons present in the outermost shell of the atom. The number of valence electrons will be same for the same group elements which is represented by Lewis dot symbol.
(c)
Answer to Problem 5.7QP
Lewis dot representation for
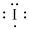
Explanation of Solution
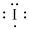
The electronic configuration of Iodine is
(d)
Interpretation:
Lewis dot symbol for the given atoms/ions must be shown
Concept Introduction:
Lewis dot symbol is used to represent the valence electrons of an atom or ion using dots surrounding the element symbol along four sides of the element symbol without maintaining exact order for the placement of dots.
In Lewis dot symbol representation, the symbol of element is surrounded by "dots" indicating the number of valence electrons available for the element. The dots can be placed one at a time on all the four sides, further electron can be placed by pairing up with the first placed dots. According to the number of electrons added or removed, charge must be placed on the Lewis dot symbol for cations and anions.
The valence electron is the number of electrons present in the outermost shell of the atom. The number of valence electrons will be same for the same group elements which is represented by Lewis dot symbol.
(d)
Answer to Problem 5.7QP
Lewis dot representation for
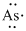
Explanation of Solution
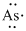
The electronic configuration of Arsenic is
(e)
Interpretation:
Lewis dot symbol for the given atoms/ions must be shown
Concept Introduction:
Lewis dot symbol is used to represent the valence electrons of an atom or ion using dots surrounding the element symbol along four sides of the element symbol without maintaining exact order for the placement of dots.
In Lewis dot symbol representation, the symbol of element is surrounded by "dots" indicating the number of valence electrons available for the element. The dots can be placed one at a time on all the four sides, further electron can be placed by pairing up with the first placed dots. According to the number of electrons added or removed, charge must be placed on the Lewis dot symbol for cations and anions.
The valence electron is the number of electrons present in the outermost shell of the atom. The number of valence electrons will be same for the same group elements which is represented by Lewis dot symbol.
(e)
Answer to Problem 5.7QP
Lewis dot representation for
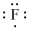
Explanation of Solution
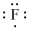
The electronic configuration of Fluorine is
(f)
Interpretation:
Lewis dot symbol for the given atoms/ions must be shown
Concept Introduction:
Lewis dot symbol is used to represent the valence electrons of an atom or ion using dots surrounding the element symbol along four sides of the element symbol without maintaining exact order for the placement of dots.
In Lewis dot symbol representation, the symbol of element is surrounded by "dots" indicating the number of valence electrons available for the element. The dots can be placed one at a time on all the four sides, further electron can be placed by pairing up with the first placed dots. According to the number of electrons added or removed, charge must be placed on the Lewis dot symbol for cations and anions.
The valence electron is the number of electrons present in the outermost shell of the atom. The number of valence electrons will be same for the same group elements which is represented by Lewis dot symbol.
(f)
Answer to Problem 5.7QP
Lewis dot representation for
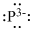
Explanation of Solution
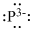
The electronic configuration of Phosphorus is
(g)
Interpretation:
Lewis dot symbol for the given atoms/ions must be shown
Concept Introduction:
Lewis dot symbol is used to represent the valence electrons of an atom or ion using dots surrounding the element symbol along four sides of the element symbol without maintaining exact order for the placement of dots.
In Lewis dot symbol representation, the symbol of element is surrounded by "dots" indicating the number of valence electrons available for the element. The dots can be placed one at a time on all the four sides, further electron can be placed by pairing up with the first placed dots. According to the number of electrons added or removed, charge must be placed on the Lewis dot symbol for cations and anions.
The valence electron is the number of electrons present in the outermost shell of the atom. The number of valence electrons will be same for the same group elements which is represented by Lewis dot symbol.
(g)
Answer to Problem 5.7QP
Lewis dot representation for
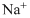
Explanation of Solution
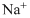
The electronic configuration of Sodium is
(h)
Interpretation:
Lewis dot symbol for the given atoms/ions must be shown
Concept Introduction:
Lewis dot symbol is used to represent the valence electrons of an atom or ion using dots surrounding the element symbol along four sides of the element symbol without maintaining exact order for the placement of dots.
In Lewis dot symbol representation, the symbol of element is surrounded by "dots" indicating the number of valence electrons available for the element. The dots can be placed one at a time on all the four sides, further electron can be placed by pairing up with the first placed dots. According to the number of electrons added or removed, charge must be placed on the Lewis dot symbol for cations and anions.
The valence electron is the number of electrons present in the outermost shell of the atom. The number of valence electrons will be same for the same group elements which is represented by Lewis dot symbol.
(h)
Answer to Problem 5.7QP
Lewis dot representation for
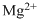
Explanation of Solution
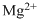
The electronic configuration of Magnesium is
(i)
Interpretation:
Lewis dot symbol for the given atoms/ions must be shown
Concept Introduction:
Lewis dot symbol is used to represent the valence electrons of an atom or ion using dots surrounding the element symbol along four sides of the element symbol without maintaining exact order for the placement of dots.
In Lewis dot symbol representation, the symbol of element is surrounded by "dots" indicating the number of valence electrons available for the element. The dots can be placed one at a time on all the four sides, further electron can be placed by pairing up with the first placed dots. According to the number of electrons added or removed, charge must be placed on the Lewis dot symbol for cations and anions.
The valence electron is the number of electrons present in the outermost shell of the atom. The number of valence electrons will be same for the same group elements which is represented by Lewis dot symbol.
(i)
Answer to Problem 5.7QP
Lewis dot representation for
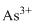
Explanation of Solution
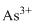
The electronic configuration of Arsenic is
(j)
Interpretation:
Lewis dot symbol for the given atoms/ions must be shown
Concept Introduction:
Lewis dot symbol is used to represent the valence electrons of an atom or ion using dots surrounding the element symbol along four sides of the element symbol without maintaining exact order for the placement of dots.
In Lewis dot symbol representation, the symbol of element is surrounded by "dots" indicating the number of valence electrons available for the element. The dots can be placed one at a time on all the four sides, further electron can be placed by pairing up with the first placed dots. According to the number of electrons added or removed, charge must be placed on the Lewis dot symbol for cations and anions.
The valence electron is the number of electrons present in the outermost shell of the atom. The number of valence electrons will be same for the same group elements which is represented by Lewis dot symbol.
(j)
Answer to Problem 5.7QP
Lewis dot representation for
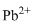
Explanation of Solution
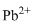
The electronic configuration of Lead is
Want to see more full solutions like this?
Chapter 5 Solutions
Chemistry: Atoms First
- 3.116 The simplest approximate chemical formula for the human body could be written as C728H4850O1970N104Ca24P16K4S4Na3Cl2Mg. Based on this formula, describe how you would rank by mass the ten most abundant elements in the human body.arrow_forward3.118 Consider common sugars such as glucose (C6H12O6) and sucrose (C12H22O11). What type of chemical bonding would you expect to find in these chemicals?arrow_forwardWhich compound of calcium is used for swimming pool water and sewage treatment? (A) Calcium hydroxide, Ca(OH)2 (B) Calcium hypochlorite, Ca(OCl)2 (C) Calcium carbonate, CaCO3 (D) Calcium oxide, CaOarrow_forward
- Write the symbol for F, Cl, Br, I, O when they react to form ions. Are they cations or anions?arrow_forwardExplaining to me in great detail how to name Sr(OH)2 and Fe(OH)3 compounds. What type of compounds are they? What are the charges on the metals? How are the nomenclature rules different for these two compounds?arrow_forwardElements in group 2A (2) of the periodic table form ions with a charge of?arrow_forward
- Ca, Cl, Na, Fe, and K. Which of these elements form cations? List their symbols with charges. Which of these elements form anions? List their symbols with charges.arrow_forwardElements in the same group of the periodic table often form oxyanions with the same general formula. The anions are also named in a similar fashion. Based on these observations, suggest a chemical formula or name, as appropriate, for each of the following ions: (a) BrO4 -, (b) SeO3 2-, (c) arsenate ion, (d) hydrogen tellurate ion.arrow_forwardWhat is the formula for iodine? For reactions involving dilute solutions of aqueous ammonia, what is the key substance involved in the reaction?arrow_forward
- Write an equation that shows the formation of a iron(II) ion from a neutral iron atom.Represent electrons as e- .If a box is not needed, leave it blank.If the coefficient is 1, do not write it.arrow_forwardWhat is a chemical bond? Explain the difference between an ionic bond and a covalent bond.arrow_forwardExplain how the atomic mass unit (amu) is defined and explain how the definition of an amu, in turn, determines the value of Avogadro’s number (NA), which is 6.0221 x 1023arrow_forward
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning




