
Concept explainers
Interpretation:
The benzophenone n-Π* triplet T1 without having benzophenone pass through its first singlet stateneeds to be determined.
Concept Introduction:
In the singlet state; the orientation of electrons has the same spin while in the triplet state; the orientation of electrons has the opposite spin.
Explanation of Solution
The reaction between benzophenone and isopropyl alcohol is shown below:
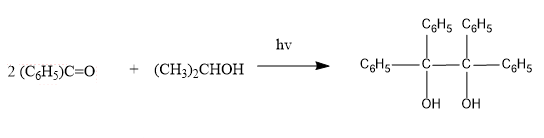
The quantum yield for the given reaction is a unity which states that each molecule of excited benzophenone gives one molecule of benzo pinacol. In the given reaction; fluorescence is not observed, a triplet excited state is involved. n-Π* triplet excited state takes a hydrogen atom from isopropanol.
The mechanism of the given reaction is shown below:
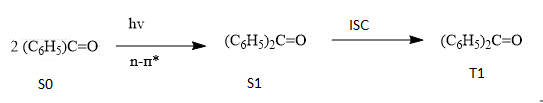
Where,
S0 = singlet ground state
S1 = first singlet excited state
T1 = first triplet excited state
ISC = intersystem crossing
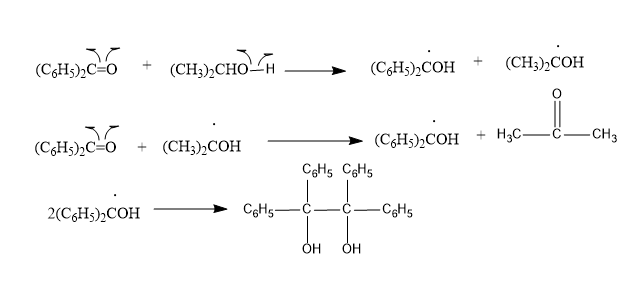
According to the mechanism; it shows that the singlet excited state of benzophenone gores through intersystem crossing to triplet state very readily. Therefore, it is the triplet state that is involved.
Want to see more full solutions like this?
Chapter 54 Solutions
EBK A SMALL SCALE APPROACH TO ORGANIC L
- 04) The proton resonance of the OH group is found at δ 5.80 for phenol and at δ 10.67 for 2-nitrophenol, both in dilute deuterated chloroform CDCl3 solution. Explain.arrow_forwardNitriles, R–=C≡N, undergo a hydrolysis reaction when heated with aqueous acid. What is the structure of the compound produced by hydrolysis of propanenitrile, CH3CH2C≡N, if it has IR absorptions from 2500–3100 cm-1 and at 1710 cm-1, and has M+=74?arrow_forwardDetermine the Ksp of the following reactions. a. NaC2H3O2 ⇄ Na+ + C2H3O2- b. HBr⇄H++Br c. Zn(OH)2 ⇄ Zn+2 + 2HO-arrow_forward
- Consider the compounds 1-pentanol,CH3-CH2-CH2-CH2-CH2-OH, melting point -78C boiling point 138C, MW=88.15. 1,6-hexanediol ,HO-CH2-CH2-CH2-CH2-CH2-CH2-OH, mp 42C,bp=250C.MW=118.18. The compound 1,6-hexanediol is more soluble in water than 1-pentanol is. This is because 1,6-hexanediol.... 1) has more carbon atoms 2)has a higher molecular weight 3)has more -OH groups 4)is a solid at room temperaturearrow_forwardThere are several isomeric alcohols and ethers of molecular formula C5H12O. Two of these exhibit the following 1H-NMR spectra. Propose a structure for each of the isomers. Isomer A: δ = 0.92 (t, 7.8 Hz, 3 H), 1.20 (s, 6H), 1.49 (q, 7.8 Hz, 2H), 1.85 (s, 1H) ppm Isomer B: δ = 1.19 (s, 9 H), 3.21 (s, 3H) ppmarrow_forwardPredict the products for the hydrohalogenation reaction of 1-methyl-1,3-cyclohexadiene with 1 equivalent of HBr in thermodynamic conditions.arrow_forward
- The proton resonance of the OH group is found at δ 5.80 for phenol and at δ 10.67 for 2- nitrophenol, both in dilute solution of deuterated chloroform CDCl3 . Explain.arrow_forwardDetermine the Ksp of the following reactions. a. NaC2H3O2 Na+ + C2H3O2- b. HBr H+ + Br c. Zn(OH)2 Zn+2 + 2HO- d. PbCl2 Pb+2 + 2Cl- e. SnSO4 Sn+2 + SO4-arrow_forwardThe following sequence of steps converts (R)-2-octanol to (S)-2-octanol. Propose structural formulas for intermediates A and B, specify the configuration of each, and account for the inversion of configuration in this sequence.arrow_forward
- Acidcatalyzed dehydration of 3hydroxy3phenylcyclohexanone leads to an unsaturated ketone. What possible structures are there for the product? At what position in the ER spectrum would you expect each to absorb? If the actual product has an absorption at 1670 cm-1, what is its structure?arrow_forwardDraw an isomer of C5H11Cl that would be expected to have four resonances in its 13C NMR spectra.arrow_forwardWhich reactions have a positive ΔSrxn? 1) 2A(g)+B(g)⟶4C(g) 2) 2A(g)+2B(g)⟶3C(g) 3) A(g)+B(g)⟶C(g) 4) 2A(g)+B(s)⟶3C(g)arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

