
(a)
The sketch of wave functions and probability density for
(a)
Answer to Problem 17P
The sketch of wave functions and probability density for
Explanation of Solution
The sketch of wave function is plotted below.
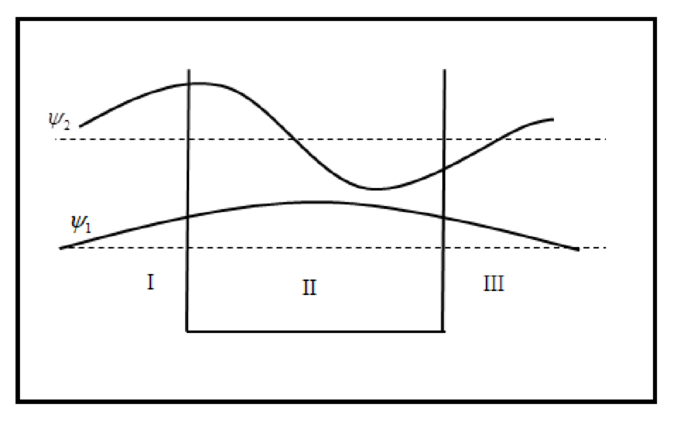
The sketch of probability density is plotted below.
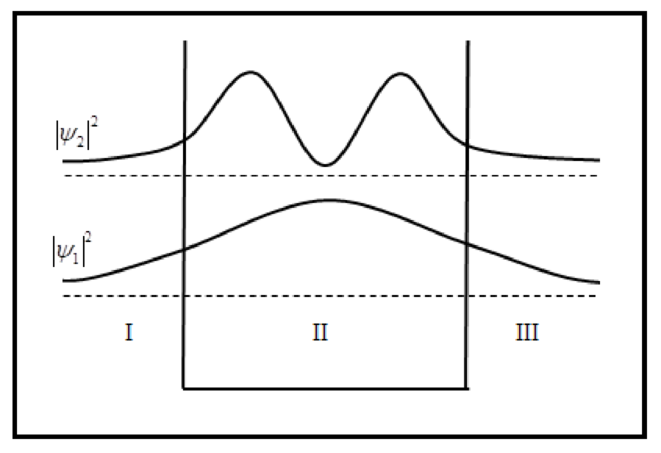
Conclusion:
The sketch of wave functions and probability density for
(b)
The probability of finding the electron between 0.15 nm and 0.35 nm for
(b)
Answer to Problem 17P
The probability of finding the electron for
Explanation of Solution
Write the expression for wave function.
Here,
Write the expression for probability.
Conclusion:
Substitute
Substitute
Thus, the probability of finding the electron for
(c)
The probability of finding the electron between 0.15 nm and 0.35 nm for
(c)
Answer to Problem 17P
The probability of finding the electron for
Explanation of Solution
Conclusion:
Substitute
Substitute
Thus, the probability of finding the electron for
(d)
The energies in electron volts for
(d)
Answer to Problem 17P
The energy for
Explanation of Solution
Write the expression for energy of particle.
Here,
Conclusion:
Substitute
Substitute
Thus, the energy for
Want to see more full solutions like this?
Chapter 6 Solutions
EBK MODERN PHYSICS
- Whether the energy gap for a system decreases, is constant, or increases as the initial quantum number for the transition increases can be determined by calculating the second derivative of the energy level expression with respect to the quantum number. Calculate the second derivative with respect to the quantum number of the energy level expression for a linear rotor, EJ = hBJ(J+1) and also for the harmonic oscillator, Ev = hυ\upsilonυ(v+1/2).arrow_forwardIf in a box with infinite walls of size 1 nm there is an electron in the energy state n=2, find its probability density, the wave function and the corresponding energy.arrow_forwardCalculate the mismatch stress in a thin epitaxial film of Ge on a Si (110) substrate. Both Si and Ge have cubic lattice structures with lattice constants 0.5431 nm and 0.5657 nm, respectively.arrow_forward
- For an electron tunneling through a rectangular barrier with a barrier height of Vo = 0.4 eV, a certain semiconductor device requires a tunneling probability of T = 10-5. If the electron energy is 0.04 eV, find the maximum barrier width.arrow_forwardCalculate the maximum permissible frequency of a crystal if you know that the mass of an atom is 8*10^¯24 and that the force constant k is equal to 1732arrow_forwardShow that in a parabolic potential well, the spacing between the energy levels is constant. In semiconductors, parabolic potential wells are often produced by using narrow square potential wells where the well to barrier width ratio gradually changes. Use the virtual crystal approximation to design a GaAs/AlAs parabolic well where the level spacing for the electron is approximately 8 meV. (Hint: This is the harmonic oscillator problem.)arrow_forward
- Calculate the threshold wave length for tungsten surface whose work function is 4.5 eV?arrow_forwardA certain semiconductor device requires a tunneling probability of T=10^-5 for an electron tunneling through a rectangular barrier with a barrier height of Vo=0.4eV. The electron energy is 0.04eV. Determine the maximum barrier width.arrow_forwardAn electron is trapped in a one-dimensional infinite potential well that is 100 pm wide; the electron is in its ground state. What is the probability that you can detect the electron in an interval of width x = 5.0 pm centered at x = (a) 25 pm, (b) 50 pm, and (c) 90 pm? (Hint: The interval x is so narrow that you can take the probability density to be constant within it.)arrow_forward
- How is duality manifested in the dual lattice structure of crystals in solid-state physics?arrow_forwardAn electron is trapped in a finite potential well that is deep enough to allow the electron to exist in a state with n= 4. How many points of (a) zero probability and (b) maximum probability does its matter wave have within the well?arrow_forwardAn electron is trapped in a one-dimensional infinite potential well that is 460 pm wide; the electron is in its ground state. What is the probability that you can detect the electron in an interval of width δx = 5.0 pm centered at x = 300 pm? (Hint: The interval δx is so narrow that you can take the probability density to be constant within it.)arrow_forward
 Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning


