
Concept explainers
MATHEMATICAL The hydrolysis of a phenylalanine-containing peptide is catalyzed by
Interpretation:
The values of
Concept introduction:
In an enzymatic reaction,
The
Answer to Problem 29RE
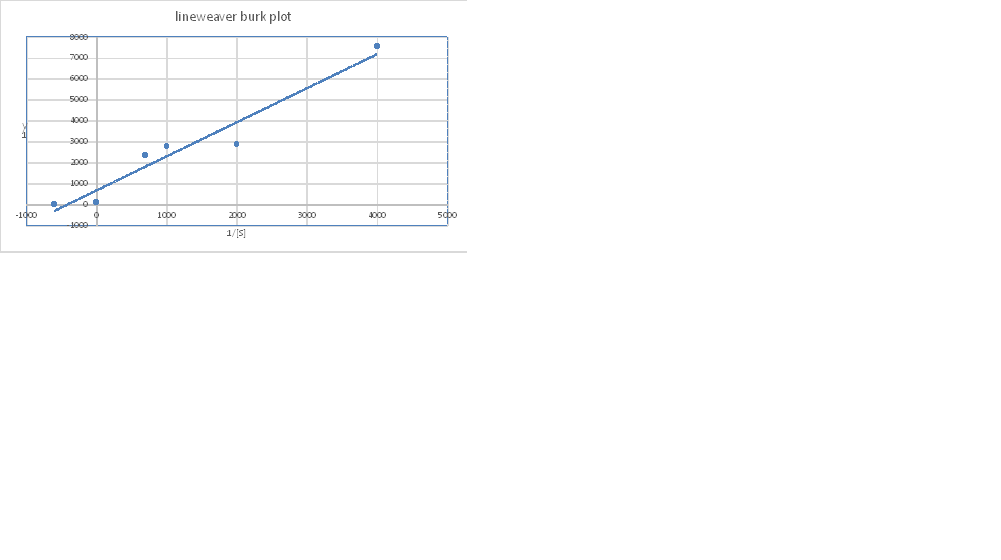
The graph shows a straight line and on extrapolating the line, it intersects X-axis as well as Y-axis, and the desired value is determined.
The value of the Y-intercept is 450, which also represents
So, the value of
Conversion of
The value of the X- intercept is -800, which represents the value of
So, the value of
Explanation of Solution
Given information:
The reciprocal of the substrate concentration and the velocity is required to draw the respective plots. Before plotting the graph, the data must be converted. The reaction velocity data is given in mole/min. It needs to be converted into mole/sec by dividing by 60.
In enzyme kinetics, the values of
This equation is further modified so that it can represent in a straight line.
Modification of the equation is,
From this equation, the values of both
With the help of the Lineweaver–Burk plot, the values of both
Want to see more full solutions like this?
Chapter 6 Solutions
EBK BIOCHEMISTRY
- REFLECT AND APPLY Why is the development of catalysis important to the development of life?arrow_forwardREFLECT AND APPLY The enzyme D-amino acid oxidase has a very high turnover number because the D-amino acids are potentially toxic. The KM for the enzyme is in the range of 1 to 2 mM for the aromatic amino acids and in the range of 15 to 20 mM for such amino acids as serine, alanine, and the acidic amino acids. Which of these amino acids are the preferred substrates for the enzyme?arrow_forwardREFLECT AND APPLY A model is proposed to explain the reaction catalyzed by an enzyme. Experimentally obtained rate data fit the model to within experimental error. Do these findings prove the model?arrow_forward
- REFLECT AND APPLY Noncompetitive inhibition is a limiting case in which the effect of binding inhibitor has no effect on the affinity for the substrate and vice versa. Suggest what a LineweaverBurk plot would look like for an inhibitor that had a reaction scheme similar to that on page 159 (noncompetitive inhibition reaction), but where binding inhibitor lowered the affinity of EI for the substrate.arrow_forwardREFLECT AND APPLY Outline the methods you would use to pro- duce human growth hormone (a substance used in the treatment of dwarfism) in bacteria.arrow_forwardMATHEMATICAL What is the ratio of concentrations of acetate ion and undissociated acetic acid in a solution that has a pH of 5.12?arrow_forward
- REFLECT AND APPLY You are studying with a friend who is describing the Bohr effect. She tells you that in the lungs, hemoglobin binds oxygen and releases hydrogen ion; as a result, the pH in- creases. She goes on to say that in actively metabolizing muscle tissue, hemoglobin releases oxygen and binds hydrogen ion and, as a result, the pH decreases. Do you agree with her reasoning? Why or why not?arrow_forwardREFLECT AND APPLY The process of protein folding is spontaneous in the thermodynamic sense. It gives rise to a highly ordered conformation that has a lower entropy than the unfolded protein. How can this be?arrow_forwardREFLECT AND APPLY You are in the process of determining the amino acid sequence of a protein and must reconcile contradictory results. In one trial, you determine a sequence with glycine as the N-terminal amino acid and asparagine as the C-terminal amino acid. In another trial, your results indicate phenylalanine as the N-terminal amino acid and alanine as the C-terminal amino acid. How do you reconcile this apparent contradiction?arrow_forward
- MATHEMATICAL Consider the reaction AB+C, where G=0.00. (a) What is the value of G (not G) when the initial concentrations of A, B, and C are 1 M, 103M,and106M? (b) Try the same calculations for the reaction D+EF, for the same relative order of concentrations. (c) Try the same calculations for the reaction GH, if the concentrations are 1Mand103M for G and H, respectively.arrow_forwardREFLECT AND APPLY What is the energy cost per amino acid in prokaryotic protein synthesis? Relate this to low entropy.arrow_forwardREFLECT AND APPLLY What are the metabolic effects of not being able to produce the M subunit of phosphofructokinase?arrow_forward
 BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
