
A fuel cell is an electrochemical device in which hydrogen reacts with oxygen to produce water and DC electricity. A 1-watt proton-exchange membrane fuel cell (PEMFC) could be used for portable applications such as cellular telephones, and a 100-kW PEMFC could be used to power an automobile
The following reactions occur inside the PEMFC:
Anode:
Cathode:
Overall:
A ?owchart of a single cell of a PEMFC is shown below. The complete cell would consist of a stack of such cells in series, such as the one shown in Problem 9.19.
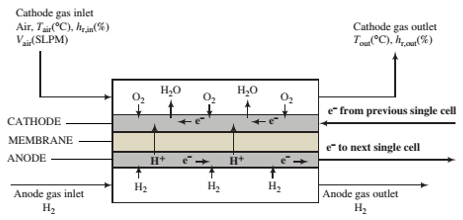
The cell consists of two gas channels separated by a membrane sandwiched between two ?at carbon-paper electrodes—the anode and the cathode—that contain imbedded platinum particles. Hydrogen ?ows into the anode chamber and contacts the anode, where H2molecules are catalyzed by the platinum to dissociate and ionize to form hydrogen ions (protons) and electrons. The electrons are conducted through the carbon ?bers of the anode to an external circuit, where they pass to the cathode of the next cell in the stack. The hydrogen ions permeate from the anode through the membrane to the cathode.
Humid air is fed into the cathode chamber, and at the cathode O2molecules are catalytically split to form oxygen atoms, which combine with the hydrogen ions coming through the membrane and electrons coming from the external circuit to form water. The water desorbs into the cathode gas and is carried out of the cell. The membrane material is a hydrophilic polymer that absorbs water molecules and facilitates the transport of the hydrogen ions from the anode to the cathode. Electrons come from the anode of the cell at one end of the stack and ?ow through an external circuit to drive the device that the fuel cell is powering, while the electrons coming from the device ?ow back to the cathode at the opposite end of the stack to complete the circuit.
It is important to keep the water content of the cathode gas between upper and lower limits. lf the content reaches a value for which the relative humidity would exceed 100%, condensation occurs at the cathode (?ooding), and the entering oxygen must diffuse through a liquid water ?lm before it cart react. The rate of this diffusion is much lower than the rate of diffusion through the gas film normally adjacent to the cathode, and so the performance of the fuel cell deteriorates. On the other hand, if there is not enough water in the cathode gas (less than 85% relative humidity), the membrane dries out and cannot transport hydrogen efficiently, which also leads to reduced performance.
A 400-cell 300—volt PEMFC operates at steady state with a power output of 36 kW. The air fed to the cathode side is at 200°C and roughly 1.0 atm (absolute) with a relative humidity of 70.0% and a volumetric ?ow rate of
(a) Explain in your own words what happens in a single cell of a PEMFC.
(b) The stoichiometric hydrogen requirement for a PEMFC is given by
(c) Use the expression of Part (b) to determine the molar rates of oxygen consumed and water generated in the unit with the given speci?cations, both in units of mol/min. (Remember that power = voltage × current.) Then determine the relative humidity of the cathode exit stream,
(d) Determine the minimum cathode inlet ?ow rate in SLPM to prevent the fuel cell from ?ooding
Learn your wayIncludes step-by-step video

Chapter 6 Solutions
ELEM.PRINCIPLES OF CHEMICAL PROCESSES
Additional Engineering Textbook Solutions
Process Dynamics and Control, 4e
Elements of Chemical Reaction Engineering (5th Edition) (Prentice Hall International Series in the Physical and Chemical Engineering Sciences)
Basic Engineering Circuit Analysis
Absolute Java (6th Edition)
Elementary Surveying (14th Edition)
Starting out with Visual C# (4th Edition)
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





